Holy crap, watching this ladysay that comparing the spike protein from the virus and the vaccine is like comparing Apples and Oranges made me steam.
Basically she says the vaccine spike is in the prefusion state so is different than the virus spike which can move from prefusion to post fusion states. So she presumes because its different its not as pathogenic as the virus spike which she concedes is dangerous. Science is broken.
She then says the burden of proof should be on those who say the vaccine spike has the same pathogenicity as the virus spike. SMH-she didn’t even giggle when she said it
Pardon me, but shouldn’t it be the responsibility for the vaccine maker to compare the pathogenicity of the vaccine spike with the virus spike. Changes can just as easily make a protein more pathogenic as it can make it less pathogenic
She also tries to confuse people by saying because the spike protein cant fuse with the cell membrane and enter the cell like the virus can there is nothing to worry about. However, we all know that the vaccine contains no spike protein. The LNP enters cells, all cells and not just those with Ace 2 receptors, , injecting the mRNA cargo in the cell which then gets transported to the ribosomes and starts churning out spike protein . The spike protein is then carried to the surface of the cell.
Furthermore, studies have shown some of the spike is ejected from the cell wrapped in exosomes and on its surface and may deliver the spike to other cells (we dont know this latter point for sure).
https://www.jimmunol.org/content/207/10/2405
The other thing she failed to mention is that unlike the virus RNA which is easily detected within the cell by Toll Like Receptors (TLR) which signals the immune system to ramp up quickly to destroy the cell, the vaccine mRNA is changed by substituting pseudouridine for uridine. This allows it to escape detection by the TLR and produce spike protein for a longer period.
Unfortunately the FDA did not require the vaccine makers to determine how long spike protein is being made, how much spike protein is being made, nor where it ends up and compare that to natural infection
She claims we should be more concerned with the spike from being infected because spike protein has been detected in people infected with SC2 and the virus makes many thousands of copies and been found all over the body
A couple of issues with this.
First off, the patients where this protein was detected (except plasma) were dead from COVID. Autopsies were done. That means they produced a lot of virus copies and due to extensive damage in the lungs it travelled throughout the body. The spike in plasma was taken from patients who were hospitalized
So we don’t know how much spike is found in the vast majority of people getting the vaccine who if infected will only have minor or no symptoms and won’t need treatment. For these people the virus is largely contained in the nasal pharynx where presumably little of the spike protein ends up in the circulation (maybe there is a study showing otherwise but I havent seen it)
One recent study showed those vaccinated with Pfizer product had as much spike in plasma as hospitalized COVID patients in the Ogata study
At least some portion of spike antigen generated after administration of BNT162b2 becomes distributed into the blood. We detected spike antigen in 96% of vaccinees in plasma collected 1–2 days after the prime injection, with antigen levels reaching as high as 174 pg/mL. The range of spike antigen concentrations in the blood of vaccinees at this early time point largely overlaps with the range of spike antigen concentrations reported in plasma in a study of acute infection (Ogata et al., 2020)
Second, up to almost 50% of people will not get infected by Sars-Cov-2 as they are naturally immune due to infection by viruses that provide them with cross reactive T-Cells and antibodies. The rest are people who will only get infected once or twice by Sars-Cov-2 , mostly with mild symptoms, and then become naturally immune which is more broad than vaccine immunity.
Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults
Here we report the virological and clinical results from the first SARS-CoV-2 human challenge study. With a low inoculum dose of 10 TCID50, robust viral replication was observed in 53% of sero- negative participants.
https://www.nature.com/articles/s41591-022-01780-9.pdf
At least six studies have reported T cell reactivity against SARS-CoV-2 in 20% to 50% of people with no known exposure to the virus
https://www.bmj.com/content/370/bmj.m3563
However, if they had their way everyone will get 4 shots of vaccines, and probably annually or biannually thereafter
Third, Pfizer did a biodistribution study on rats. They used the LNP with some other mRNA so did not look for spike. However, they said it was assumed the mRNA would end up where the LNP was detected, which was pretty much everywhere
Lastly, the spike protein seems to stick around longer in the lymph nodes than in those with natural infection
The observed extended presence of vaccine mRNA and spike protein in vaccinee LN GCs for up to 2 months after vaccination was in contrast to rare foci of viral spike protein in COVID-19 patient LNs.
Here are some other studies related to spike protein. The burden of proof is on those pushing the vaccines. Hopefully they will have a chance to present it to a Grand Jury
Ab2 antibodies could also mediate neurologic effects of SARS-CoV-2 infection or vaccines, given the expression of ACE2 on neuronal tissues, the specific neuropathologic effects of SARS-CoV-2 infection,and the similarity of these effects to Ab2-mediated neurologic effects observed in other viral models.
😅Translation: Ab2 antibodies to spike in this case are specific for ACE2 which decrease ACE2 activity. This could lead to an increase in the abundance of Ang II, which causes a proinflammatory state that triggers symptoms of PASC(Long Covid)
https://www.nejm.org/doi/full/10.1056/NEJMcibr2113694
S1 protein in the plasma of nurses after vaccination for up to 28 days after vaccination, and also the entire spike protein for a shorter period
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciab465/6279075
Evidence for SARS-CoV-2 Spike Protein in the Urine of COVID-19 Patients
https://kidney360.asnjournals.org/content/2/6/924
New-Onset Kidney Diseases after COVID-19 Vaccination: A Case Series
https://www.ncbi.nlm.nih.gov/pmc/a
The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147-receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease
https://www.biorxiv.org/content/10.1101/2020.12.21.423721v2.full.pdf
Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) Up to 15 Months Post-Infection
https://www.biorxiv.org/content/10.1101/2021.06.25.449905v1.full
Implications of the SARS-CoV-2 spike protein interaction with type-1 macrophages via α7-nAChR
https://www.qeios.com/read/26GTOD.2
SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19
https://www.medrxiv.org/content/10.1101/2021.03.05.21252960v1
Free SARS-CoV-2 Spike Protein S1 Particles May Play a Role in the Pathogenesis of COVID-19 Infection
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7772528/
The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier
https://pubmed.ncbi.nlm.nih.gov/33053430/
The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice
https://www.nature.com/articles/s41593-020-00771-8.pdf
Domains and Functions of Spike Protein in SARS-Cov-2 in the Context of Vaccine Design
https://res.mdpi.com/d_attachment/viruses/viruses-13-00109/article_deploy/viruses-13-00109-v3.pdf
Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2660061/#__ffn_sectitle
Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: implications for the vaccine
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7499017/
https://pubmed.ncbi.nlm.nih.gov/33300001/
And lastly, something the virus doesn’t have, the LNP
Nonclinical Evaluation Report
BNT162b2 [mRNA] COVID-19 vaccine (COMIRNATYTM)
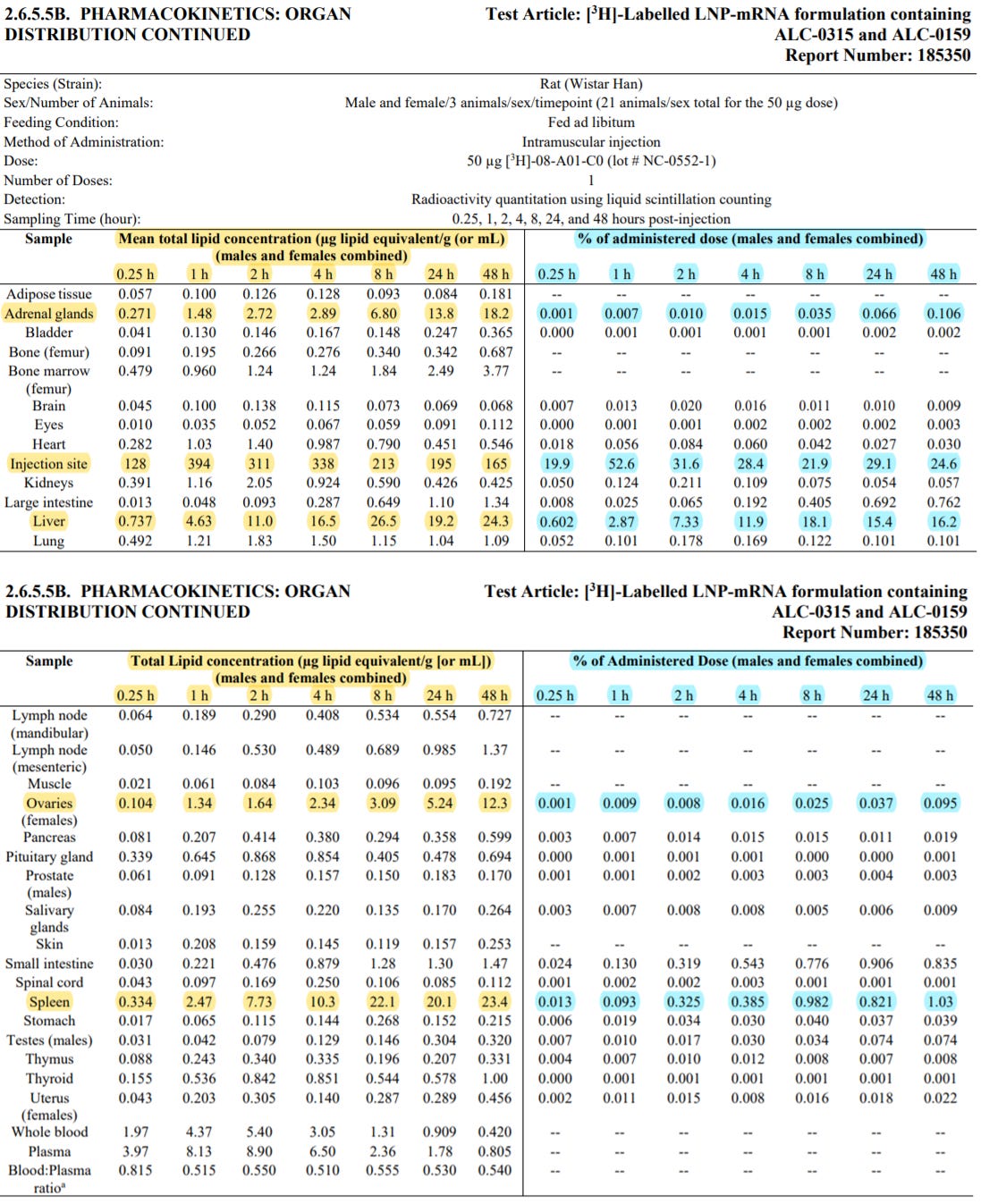
The biodistribution of the mRNA and expressed antigen encoded by the mRNA component of BNT162b2 is expected to be dependent on the LNP distribution. To visualise the tissue distribution of the mRNA-LNP formulation, mRNA encoding luciferase was formulated in an LNP formulation (LNP8) similar to the BNT162b2 vaccine. Following an IM injection of the luciferase mRNA formulation in mice, luciferase was detected by whole body imaging mainly at the injection site, which declined to the background level after 9 days. Luciferase was also seen in liver, which disappeared in 48 hours. This is consistent with other kinetics studies with mRNA-LNPs (Pardi et al. 2015). Sensitivity of the imaging detection system was low. Distribution to other tissues, e.g. draining lymph nodes, is highly likely (Lindsay et al. 2019), but the level was probably below the limit of detection of the imaging system.
The distribution of lipid nanoparticles encapsulating mRNA encoding luciferase was investigated by monitoring of a radiolabelled lipid-marker after a single IM injection to Wistar rats. Major uptake of the lipid marker, probably representing the lipid nanoparticles, was noted in the injection site and liver with low distribution in spleen, adrenal glands and ovaries (distribution was not investigated in draining lymph nodes). The total radioactivity recovery was very low at all time-points (range = 20 – 60%), possibly because the draining lymph nodes of the injection site were not collected and faeces, urine, carcass and cage-wash samples were not analysed. The Sponsor stated that the tissues collected for this study were a standard panel of tissues, which did not include the draining lymph nodes.
Doses higher (50 and 100 μg mRNA/animal) than those proposed in humans (30 μg mRNA) were tested in rats (avg. BW ~ 225 g), which showed clinical signs of piloerection, hunched body, decreased activity and irregular respiration. This might indicate toxicity of the LNP formulation at high doses. The 50 μg mRNA/animal dose is ~ 370 × the proposed human dose on a μg/kg BW basis.
In summary, the limited pharmacokinetic studies indicate that the vaccine LNP formulation is expected to deliver the mRNA effectively in vivo and the antigen expressed mainly at the injection site, liver and probably in draining lymph nodes.
The limited studies showed slow elimination of ALC-0315 and retention in liver, and complete elimination of ALC-0159 in 14 days, with the latter eliminated in faeces most likely by biliary excretion.