
As many know, CDC was forced to release the V-Safe data which showed that 7.7% had to seek medical care after vaccination. 25% missed work, school, or had bad reactions to the vaccine.
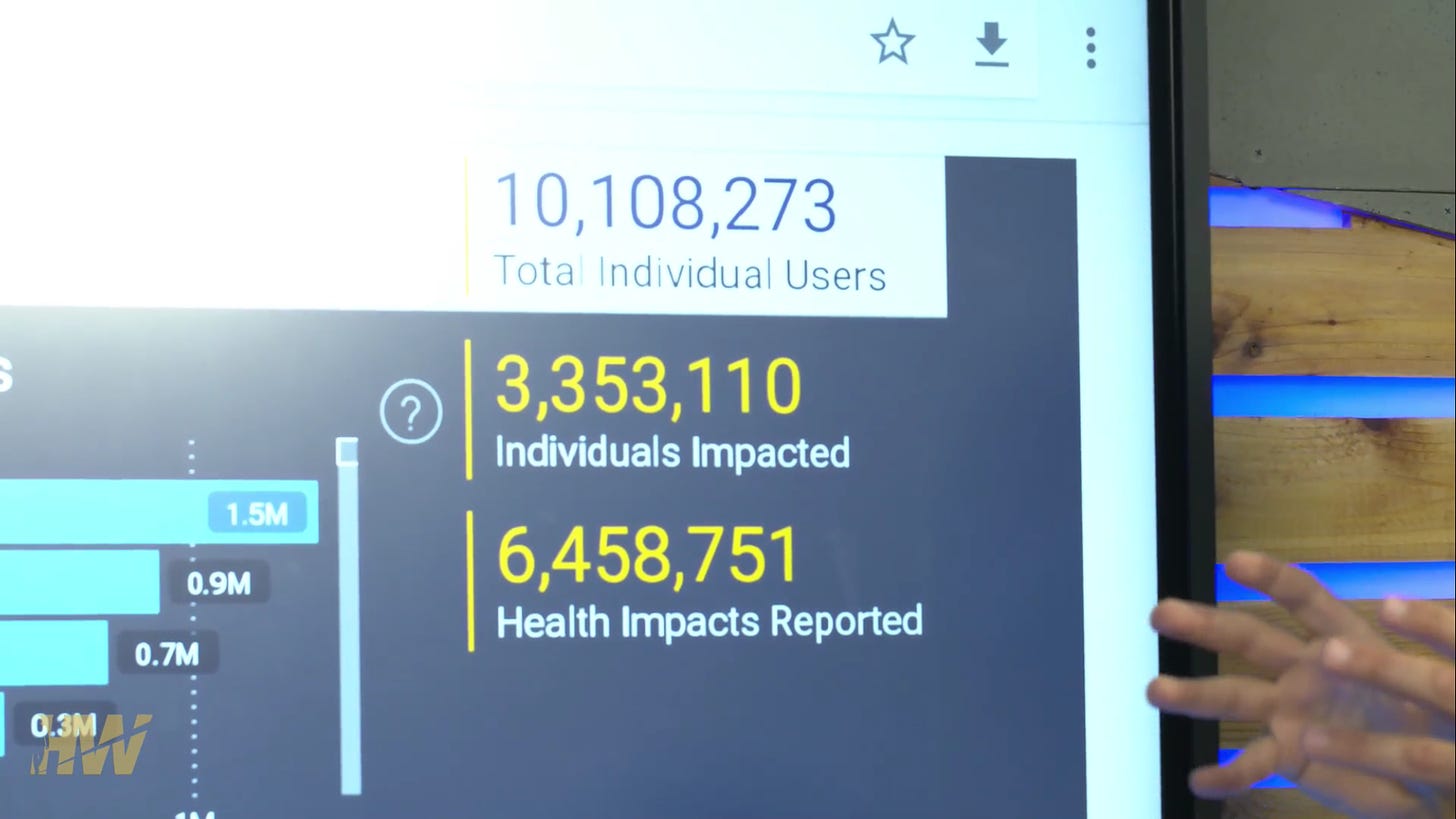
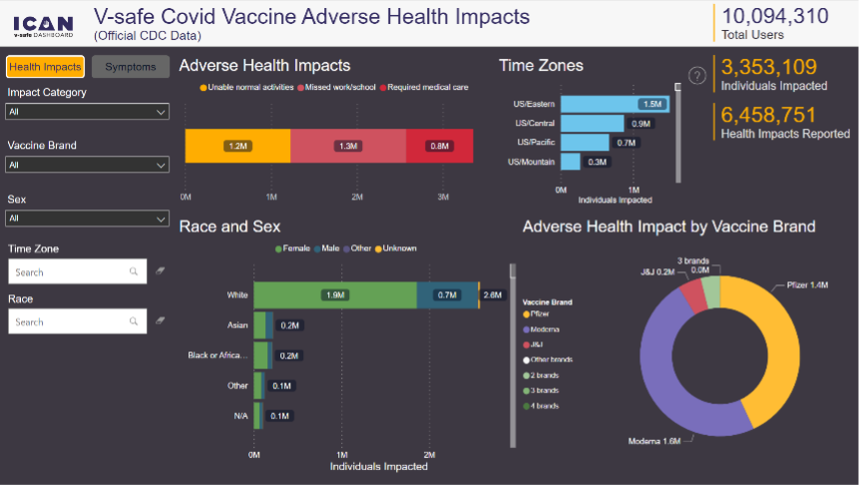
Of the 10,108,273 users who participated in V-Safe the following was found (you can review the data yourself here, keep in mind there will always be a degree of underreporting that occurs):
•1,225,867 (12.13%) were unable to perform their normal activities.
•1,344,330 (13.3%) missed work or school.
•782,913 (7.7%) required medical care, on average 2.7 times.
They eye opener here is 7.7% requiring medical care.
64% of users reported no health impact
They allow a measly 18 adverse events to be reported by users as opposed to the over 25,000 MedDRA PT codes (25,592 to be precise) used in the VAERS context of which over 14,000 have been reported in the context of the COVID-19 injections.
CDC spent 463 days stonewalling ICANS attorney Aaron Siri in his efforts to obtain the data and make it public
A
B
C
The problem with the data as presented above is it appears people were submitting the same symptoms multiple times, perhaps after each dose, and of course, many had multiple symptoms.
The only data released by CDC direct to the public on V-Safe , at least the only report that I could find, came in January 2021 just as Rochelle Walensky was taking control with the Biden Administration
V-safe is a safety monitoring system established by CDC specifically for the COVID-19 vaccination program. V-safe participants voluntarily self-enroll and receive smartphone text messages providing hyperlinks to web surveys.** During the first week after vaccination, enrollees complete daily surveys asking about local injection site and systemic reactions. Enrollees are asked if they missed work, were unable to perform normal daily activities, or received care from a medical professional because of reported symptoms or heath conditions. Enrollees who report seeking medical care are contacted, and a VAERS report is completed if clinically indicated. Persons who do not report their sex as male are asked about pregnancy status at time of vaccination (initial survey) and about a positive pregnancy test result (3- and 6-week surveys); reported pregnancies are followed up through the v-safe pregnancy registry.††
CDC conducted descriptive analyses of data from VAERS and v-safe during December 14, 2020–January 13, 2021, a period when the first and second doses of Pfizer-BioNTech vaccine and the first dose of Moderna vaccine were administered. Because LTCF staff members were vaccinated at LTCF facilities, residents of LTCFs were presumptively identified by restricting examination of VAERS reports to adults aged ≥65 years with a documented vaccination at an LTCF. To ensure that LTCF residents with serious adverse events were identified, manual review was conducted of all reports of serious adverse events among those vaccinated in LTCFs, regardless of vaccine recipient’s age. Administered vaccine doses were reported to CDC.§§ These activities were reviewed by CDC and are consistent with applicable federal law and CDC policy.¶¶ All analyses were conducted using SAS software (version 9.4; SAS Institute).
During December 14, 2020–January 13, 2021, a total of 13,794,904 COVID-19 vaccine doses were administered in the United States; 8,436,863 (61.2%) doses were administered to women. VAERS received 6,994 reports of COVID-19–associated adverse events during this period. Among all reports, 6,354 (90.8%) were classified as nonserious and 640 (9.2%) as serious, including 113 (1.6%) deaths. Headache (22.4%), fatigue (16.5%), and dizziness (16.5%) were the most frequently reported symptoms after vaccination with either vaccine (Table 1).
v-safe Reports
During December 14, 2020–January 13, 2021, v-safe enrolled 1,602,065 vaccine recipients who completed at least one survey; 814,648 (50.8%) received Pfizer-BioNTech, and 787,417 (49.2%) received Moderna vaccines. The median recipient age was 46 years (range = 16–110 years); 1,106,656 (69.1%) were women. There were 10,825 (0.68%) enrollees who reported that they were pregnant at the time of vaccination, and 262 (0.02%) reported a positive pregnancy test result after vaccination.
Enrollees reported more reactions on the day after vaccination than on any other day. For the Pfizer-BioNTech vaccine, reactions were more frequent after the second dose than the first; the reported rate of fever and chills was more than four times higher after the second dose than after the first (Table 2).
TABLE 2. Percentage of v-safe enrollees who completed at least one survey (N = 1,602,065) with local and systemic reactions reported for day 0–7 and for day 1 after receiving Pfizer-BioNTech and Moderna COVID-19 vaccines — v-safe,* United States, December 14, 2020–January 13, 2021
Local and systemic reaction
Percentage of v-safe enrollees reporting reactions
Both vaccines
Injection site pain
70.9%
Fatigue
33.5%
Headache
29.5%
Myalgia
22.9%
Chills
11.6%
Fever
11.4%
Injection site swelling
10.8%
Joint pain
10.4%
Nausea
8.9%
https://www.cdc.gov/mmwr/volumes/70/wr/mm7008e3.htm
From the above data CDC presented we can see excluding injection site pain 66.5% did not report any symptoms. This is consistent with the data just released to ICAN (64%)
It should be noted most of that early data was 1 shot, symptoms are more frequent after the 2nd shot
In any event, since that first report its been Crickets. Rochelles strategy since taking over is Lie, Lie, Lie and Cover Up the Data on top of the Lies
This does not mean they did not use V-Safe to provide support for whatever conclusions they wanted to reach to mislead us over the vaccine safety.
This article explains
And what has v-safe shown so far? “The findings in normal, regular people that got the vaccine were pretty reflective of what you saw in the clinical trials,” said Vanderbilt’s Edwards. Edwards also served on an independent safety data monitoring committee for the Pfizer-BioNTech vaccine, now branded as Comirnaty.
Unlike VAERS, v-safe data is not published without context. Meaning, no one can just sort through the database and interpret the numbers as they please, as many do with VAERS data. It is, however, publicly shared through CDC studies and presentations given during meetings held by the CDC’s independent panel of experts, the Advisory Committee on Immunization Practices.
And like VAERS reports, v-safe data is susceptible to misinterpretation.
Information gleaned from v-safe has been used in several safety analyses, including one focused on adolescents. That analysis, published Aug. 6, found that serious adverse events are rare among adolescents, partly based on v-safe surveys from tens of thousands of people ages 12 to 17. The analysis also found that a minority reported being unable to perform “normal daily activities” the day after receiving a second dose.
[Approximately 129,000 U.S. adolescents aged 12–17 years enrolled in v-safe after Pfizer-BioNTech vaccination; they reported local (63.4%) and systemic (48.9%) reactions with a frequency similar to that reported in preauthorization clinical trials......
Fewer than 1% of adolescents aged 12–17 years required medical care in the week after receipt of either dose; 56 adolescents (0.04%) were hospitalized]*
https://www.cdc.gov/mmwr/volumes/70/wr/mm7031e1.htm
[*note-only 1% adolescents required medical care. It would be nice to have an age breakdown for the 7.7% that required medical care in the ICAN data dump]
Who Is Participating in V-Safe?
More than 9.2 million people have enrolled in v-safe as of Aug. 9, or roughly 5% of the U.S. population who received at least one dose of a covid vaccine. This seemingly low participation rate is often linked to weak advertising and public education programs about v-safe. Also, a segment of the vaccinated public likely considered it tedious or had privacy concerns. The number also excludes people who do not have smartphones.
V-safe has perhaps been most helpful at providing real-world evidence that the covid-19 vaccines are safe during pregnancy. This is important because there was little information on how the vaccines affected pregnancy when they were first authorized, said Dr. Dana Meaney-Delman, a member of the CDC’s vaccine task force, in a recent call with clinicians.
Pregnant women were excluded from the initial clinical trials that led to the emergency use authorization of the Pfizer, Moderna and J&J vaccines, and misinformation was rampant.
Because pregnant health care workers got vaccinated and enrolled in v-safe, Meaney-Delman said, there is more evidence that indicates the benefits of getting vaccinated during pregnancy outweigh any potential risks.
Following the publication of an analysis that leaned on v-safe’s vaccine pregnancy registry, the CDC recommended on Aug. 11 that people who are pregnant, lactating or trying to become pregnant get vaccinated against covid.
Currently, uptake is low — as of mid-August, 23% of pregnant people ages 18 to 49 are at least partially vaccinated.
As mentioned in the above article V-Safe collected information on pregnancies. Has CDC bothered to show the public this data that they have paid for? Nada. At least I haven’t seen it
V-safe Pregnancy Registry
Updated July 18, 2022
Participation in the Registry
CDC invites people who received any dose of COVID-19 vaccine in the periconception period (within 30 days before last menstrual period) or during pregnancy to participate in the v-safe COVID-19 Vaccine Pregnancy Registry.
Pregnant people who would like to participate must be enrolled in v-safe.
If people enrolled in v-safe report that they were pregnant at the time of vaccination or became pregnant shortly after vaccination, the registry staff* may call them to learn more about their pregnancy course and outcome.
Even if you are no longer pregnant, you may still be eligible to enroll in the registry.
V-safe and the V-safe COVID-19 Vaccine Pregnancy Registry: What’s the Difference?
v-safe is a smartphone-based system that uses text messaging and web surveys to provide personalized health check-ins after you receive a COVID-19 vaccine. The v-safe COVID-19 Vaccine Pregnancy Registry is for v-safe participants who self-identify as pregnant at the time of vaccination or shortly thereafter (within 30 days of vaccination). The registry activities are in addition to the v-safe after vaccination health check-ins that participants receive via text message.
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafepregnancyregistry.html
We need to see this data and any analysis of the data by CDC
Apparently the app has a free form section where the patient can describe their symptoms or diagnosed. This has not been released yet. CDC should also have generated internal summaries and analysis of the collected data. Not released.
Furthermore each V-Safe report should have resulted in CDC preparing a VAERS Report or associating that V-Safe data with a VAERS Report generated by the patient or their medical provider. They should release all VAERS Reports that were related to V-Safe participants reports.
I know many of you are pinning your hopes on Republicans in the upcoming elections. But how many of them have taken a stand on vaccine safety and improving vaccine data transparency?
No doubt there are a few, but make sure you pin your hopes on someone who speaks clearly on the issue



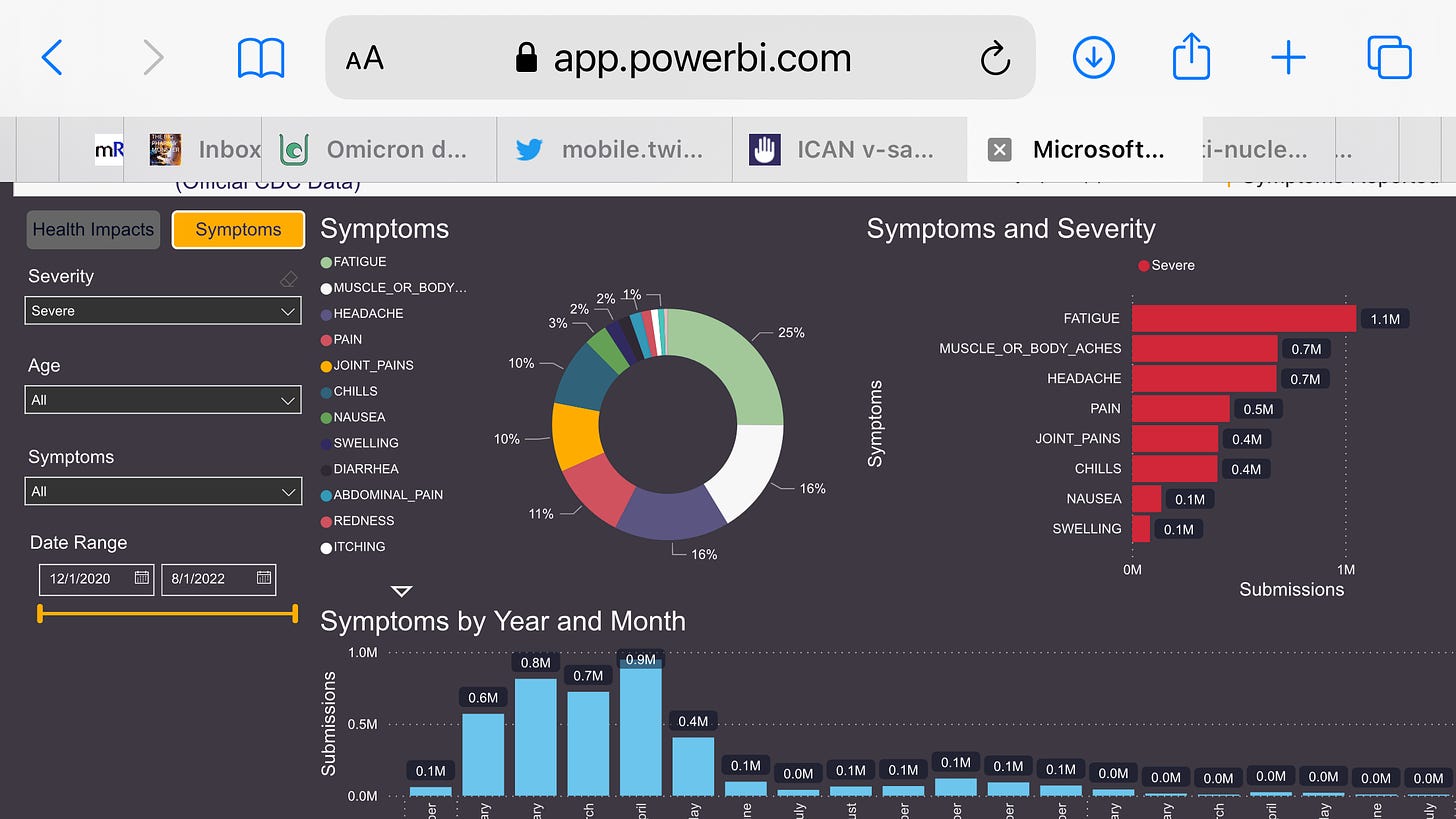
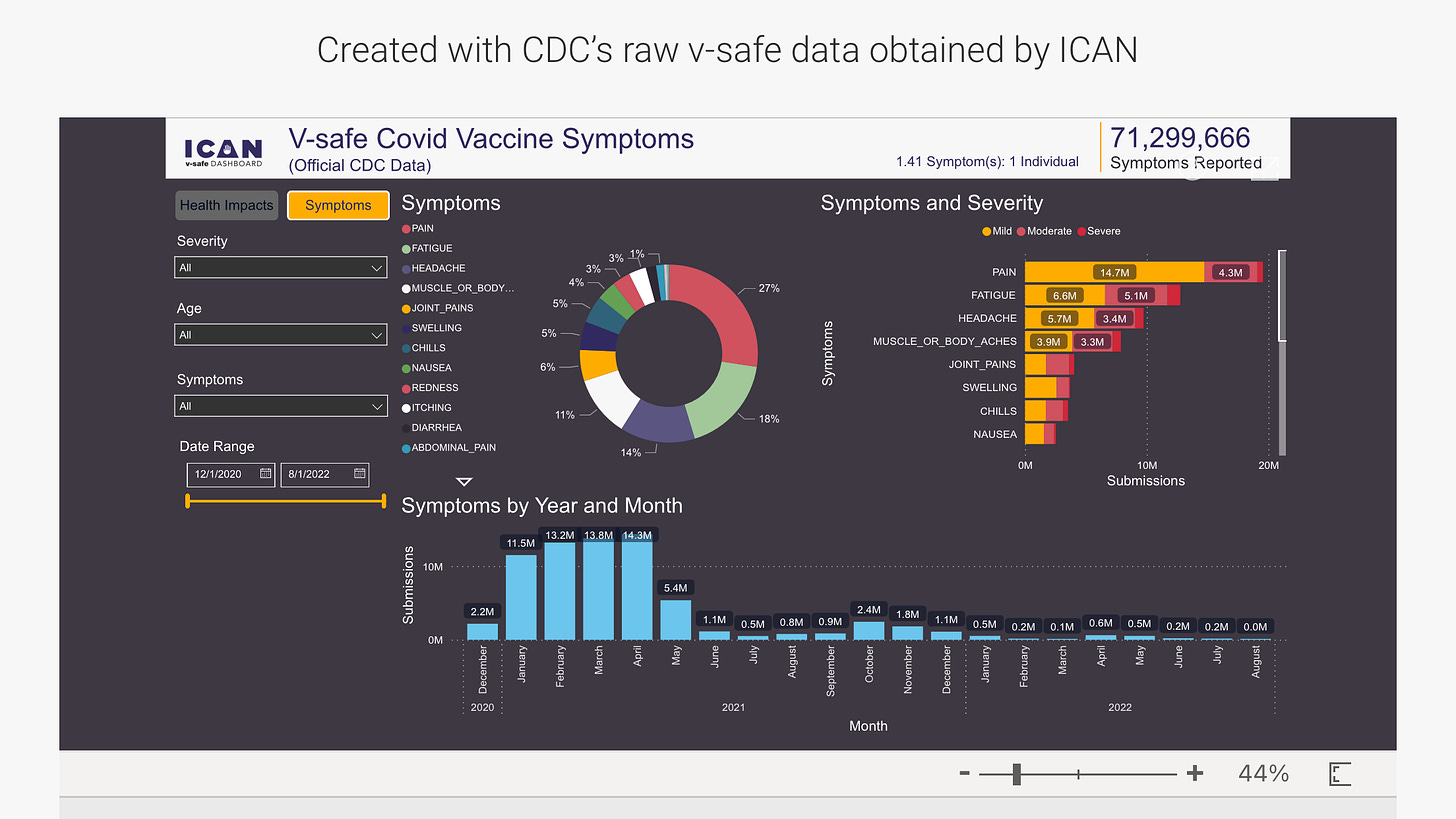




FWIW I have started wading through the csv files themselves — so far expect analyses of this will by definition come to benign conclusions. As self reported data, it by definition cannot contain debilitating severe events. (also if fields in the CSV are any indication, possible there is no option to enter that in the app — could be a built in bias)
VAERS or tying external data to these (which CDC can in principle do, and technically possible with the existing anonymization, but won’t do unless compelled) is going to be required to normalize/compare to VAERS I’m guessing.
The big time streamed event data could be interesting, but takes work & head scratching.