Interesting study here
https://www.medrxiv.org/content/10.1101/2022.04.02.22273333v1.full
Conclusion: We report high incidence of omicron infections despite recent booster vaccination in triple vaccinated individuals. Vaccine-induced antibody titres seem to play a limited role in risk of omicron infection. High viral load and secretion of live virus for up to nine days may increase transmission in a triple vaccinated population.
Remarkably, 22% of study participants became infected during the study period, with no significant impact of pre-infection antibody titers.
Viral RNA trajectories were similar and suggestive of infectivity by all omicron sublineages, implying that three vaccine doses offer limited protection against BA.1, BA.1.1 and BA.2 infections and onward transmission.
SARS-CoV-2 anti wild-type (WT) IgG, cross-reactive IgG capable of binding omicron (BA.1, BA.1.1 and BA.2), and IgG capable of blocking WT and omicron (BA.1, BA.1.1 and BA.2) spike ACE2 binding (surrogate neutralization), were measured in post vaccination samples drawn at start of the screening study (V-PLEX SARS-CoV-2 Panel 22, 23 and 25, Meso Scale Diagnostics, Maryland, USA)
SARS-CoV-2 recovered vaccinees showed stronger antibody responses than SARS-CoV-2 naïve vaccinees following primary vaccination
Consistent with recent reports , post booster neutralization of omicron BA.1 was substantially lower compared to neutralization of WT both in SARS-CoV-2 naïve participants (10.2 fold-change, p<0.001) and in SARS-CoV-2 recovered participants (fold change 19.2, p<0.0001)
61 participants who were negative at first sample and subsequently tested positive during the four week screening were enrolled in a 14-day follow up with self-administered samples every second day
Analysis of viral RNA levels revealed a peak day three after initial positive test, and that the majority (91%) of the participants were positive with Ct < 30 nine days after initial positive test.
Six (9%) participants, all with Ct values >30 in the initial positive sample, were qPCR negative in all follow-up samples.
23 of 61 (38%) participants remained asymptomatic > 48 hours after first qPCR-positive sample, with a median pre-symptomatic Ct value of 28.9 (range 19.4-38).
Six participants (9%) remained asymptomatic throughout the whole course of their infection
Peak viral load and time to viral clearance was not significantly different between participants with asymptomatic course of infection and those with symptoms at any time point during the infection (p=0.06 and p=0.095, respectively).
Among the 55 participants with symptomatic infection (91%), “common cold” symptoms dominated.
Presence of symptoms at time of sampling correlated to higher viral load compared to samples from asymptomatic participants (p<0.0001)
Fever, cough, headache and anosmia at any time point throughout the course of infection were associated with an increased viral load
23.0% of the SARS-CoV-2 naïve (n=50) and 20.4% of the SARS-CoV-2 recovered (n=32) participants became infected.
When comparing cross-reactive antibody responses capable of neutralizing omicron sublineages we found slightly but significantly higher titers capable of neutralizing BA.2 as compared to BA.1
[me: this is strange, why would a virus evolve to be more susceptible to being neutralized in a highly vaccinated population, shouldn’t it move to escape neutralization from vaccine created antibodies?]
WGS (whole genome sequencing) was successful in 71/72 cases .....26 BA.1 (of which 20 were included in follow-up), 21 BA.1.1 (13 in follow-up), and 24 BA.2 (22 in follow-up) infections.
Notably, pre-infection anti-WT spike IgG were comparable in participants that subsequently became infected, and those that remained qPCR negative, regardless of omicron sublineage
Median Ct value of first positive sample was 29.4 in BA.1 vs. 25 in BA.2 infections corresponding to an approximate 100-fold higher level of viral RNA in BA.2 infected individuals early in the course of infection.
[me: even more strange given vaccine antibodies showed more affinity for BA.2, could this be evidence the antibodies are facilitating infection/replication. This would explain its natural selection?]
There was also a trend towards a longer time to viral clearance in BA.2 infections as compared to BA.1 infections (p=0.13) . Symptom duration was significantly longer in BA.2 infected compared to BA.1 (median duration of symptoms 8 vs 6 days), p<0.01
Our findings emphasize that vaccine-induced antibody titres play a limited role in omicron infection risk prediction.
[me: This new study may shine more light on if Vaccine induced antibodies may be enhancing infection and viral load/duration]
https://www.nature.com/articles/s41586-022-04702-4_reference.pdf
About 6% of blood monocytes in COVID-19 patients are infected with SARS-CoV-2.Monocyte infection depends on uptake of antibody-opsonized virus by Fcγ receptors. Vaccine recipient plasma does not promote antibody-dependent monocyte infection. SA
[me: Bruce Patterson detected intermediate and non-classical monocytes containing S1 protein for as long as 15 months after infection. Non-Classical Monocytes crawl along the blood vessel walls repairing them but the spike protein or S1 part can cause inflammation and symptoms
https://www.biorxiv.org/content/10.1101/2021.06.25.449905v3]
Many intermediate (~60%) and non-classical (~40%), but none of the more abundant classical, monocytes had taken up SARS-CoV-2 virussince they stained for nucleocapsid (N) .
Since only monocytes that expressed FcγRIIIa (CD16), an important mediator of antibody-dependent phagocytosis, took up virus, anti-spike RBD IgG plasma titers were measured in 64 COVID-19 plasma samples obtained at ED presentation, 20 HD (Healthy Donors) and 5 patients who presented with COVID-19-like symptoms but were SARS-CoV-2 PCR- (non-COVID-19 patients).
Most COVID-19 patients, but not HD or non-COVID-19 controls, had elevated anti-spike RBD IgG, suggesting that they had been infected for approximately a week.
SARS-CoV-2 begins to replicate in monocytes, but infection is aborted, and infectious virus is not detected in infected monocyte culture supernatants.
Instead, infected cells undergo inflammatory cell death (pyroptosis) mediated by activation of NLRP3 and AIM2 inflammasomes, caspase-1 and GSDMD.
Moreover, tissue-resident macrophages, but not infected epithelial and endothelial cells, from COVID-19 lung autopsies have activated inflammasomes. These findings taken together suggest that antibody-mediated SARS-CoV-2 uptake by monocytes/macrophages triggers inflammatory cell death that aborts production of infectious virus but causes systemic inflammation that contributes to COVID-19 pathogenesis.
dsRNA and NG detection strongly suggested that monocytes replicate SARS-CoV-2. To confirm viral replication and further assess whether uptake is ACE2-mediated, HD monocytes were infected in the presence of COVID-19 plasma and the antiviral drugs, Remdesivir, an inhibitor of the viral RNA-dependent RNA polymerase, and Camostat mesylate, an inhibitor of TMPRSS2, which primes the spike protein for ACE2-mediated entry37 (Fig. 4l, m, Extended Data Fig. 5e–g).
Monocyte infection, assessed by N or NG positivity, was unaffected by Camostat, but significantly and comparably inhibited by Ig depletion or Remdesivir, confirming antibody-dependent entry and viral replication.
Lack of inhibition by Camostat and anti-ACE2 suggests that ACE2 is unlikely to be a dominant receptor for viral entry into monocytes but does not rule out a minor role in monocyte infection or a more prominent role in infection of ACE2+ macrophages.
Although multiple assays indicated monocytes begin viral replication, we next assessed whether infected monocytes produce infectious virus.
Infectious SARS-CoV-2 is detected in COVID-19 plasma only with especially sensitive assays, and we did not detect infectious virus by plaque assay in COVID-19 plasma samples.
Although infected HD monocyte culture supernatants formed plaques in Vero cells when culture supernatants were harvested immediately after infection (likely detecting input virus), no infectious virus was detected when culture supernatants were harvested 48 hours post infection (hpi) By contrast plaques were easily detected in culture supernatants from infected Vero harvested 48 hpi. Thus, monocyte infection did not produce infectious virus.
Most dying monocytes in COVID-19 blood had activated inflammasomes, suggesting that monocytes are dying of pyroptosis.
It may be surprising that monocyte infection and cell death has not been widely recognized. However, this may be because (1) many COVID-19 studies use thawed, frozen cells, and dying cells do not survive freeze-thawing, (2) published studies have not looked at whether circulating mononuclear cells are dying, and (3) few researchers have looked for monocyte infection because monocytes do not express ACE2.
FcγR-mediated uptake of antibody-coated virus into monocytes is a double-edged sword. Pyroptosis, which occurs rapidly, likely aborts viral infection before infectious virions are fully assembled. Monocyte/ macrophage infection is a dead end for the virus - it removes virions from the extracellular milieu, blocks them from producing infectious progeny and prevents them from disseminating.
Pyroptosis in infected monocytes/macrophages also sounds a potent immune alarm to recruit and activate innate and adaptive immune cells to infection sites to mobilize immune defense.
On the other hand, the inflammatory mediators spewed out from pyroptotic monocytes and macrophages can cause cytokine storm.
It may not be a coincidence that clinical deterioration coincides temporally with the detection of SARS-CoV-2 antibody responses. In fact, some recent studies suggest that higher anti- body titers correlate with disease severity
.Our findings, which implicate opsonizing antibodies in mono- cyte infection and inflammasome activation, suggest that antibod- ies may contribute to deleterious immune reactions associated with severe disease.
FcγR-mediated monocyte infection is an example of antibody-mediated enhancement (ADE) of infection.
[me: Now for the obligatory praise for Vaccines necessary to get published]
Nonetheless, overwhelming evidence shows that vaccine-generated neutralizing antibod- ies prevent infection and improve clinical outcome of breakthrough infections, suggesting that anti-spike antibodies are highly beneficial.
Plasma from vaccinated individuals did not promote monocyte infection, indicating that ADE is not a concern with respect to vaccination. [me: Authors still engaging in damage control to get past censors-no evidence for this is presented in the paper]
Therapeutically administered anti-spike neutralizing monoclonal anti- bodies, however, only improve clinical outcome if given early, before hospitalization, and antibody-containing convalescent sera have not shown clinical benefit.
Thus, it is worth considering whether some antibodies might have both protective and deleterious effects.
Antibodies are clearly beneficial for blocking infection of ACE2-expressing lung and airway epithelia, where the virus completes replication to produce infectious progeny.
However, antibody properties that affect FcR-mediated cellular uptake, phagocytosis, cytotoxicity and complement activation, can affect disease pathogenesis .
Early development of afucosylated anti-spike antibodies promotes alveolar macrophage inflammation and is associated with COVID-19 severity .
Afucosylated antibodies are increased during acute infection with enveloped viruses like SARS-CoV-2 but are not abundant after COVID-19 vaccination or other types of antigen exposure [me: we will review this below]
IgG isolated from COVID-19 patients with a higher proportion of afucosylated antibodies significantly, but weakly, increased in vitro monocyte infection but IgG from patients with fewer afucosylated antibodies did not.
The increased pathogenicity of afucosylated antibodies could be secondary to antibody-mediated infection and downstream inflammasome activation in monocytes and macrophages.
However, our findings about afucosylation are preliminary and more work is needed to make this association. Characterizing how antibody features, such as afuco- sylation, sialylation and choice of constant region, alter protective vs deleterious functions of anti-spike antibodies will be important not only for understanding SARS-CoV-2 pathogenesis, but also for choosing the best preparations of convalescent patient plasma and monoclonal antibodies for therapy and/or prevention of severe disease.
[me: From the study below convalescent plasma did more harm it seems and while not conclusive suggest the possibility of ADE]
https://www.nejm.org/doi/10.1056/NEJMoa2103784
“Five patients in the plasma group and 1 patient in the placebo group died. Outcomes regarding worst illness severity and hospital-free days were similar in the two groups.
The administration of Covid-19 convalescent plasma to high-risk outpatients within 1 week after the onset of symptoms of Covid-19 did not prevent disease progression.”
[me: The study below seems to confirm vaccines dont produce afucosylated antibodies when measured 28 days after the 1st dose
Funders are Zuckerberg Foundation, NIAID, Big Pharma, etc
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8939764/#__ffn_sectitle
This study below does not seem to agree with the above linked study, at least not for naive antigen experienced vaccinated persons (not previously infected or sero-converted). This is because the earliest antibodies before 28 days have a higher percentage of afucosylated antibodies]
https://www.biorxiv.org/content/10.1101/2022.02.14.480353v1
The findings reveal that the level of afucosylated anti-spike antibodies increases transiently in infection-naïve individuals after the first vaccine dose.
N-glycosylation of the antibody’s Fc region is required for mediating effector functions. The galactose and fucose residues present in the Fc N-glycan core play vital roles in modulating the activity of Fc-gamma receptors on natural killer cells and myeloid cells, respectively.
Fc-gamma receptors are membrane molecules that bind the Fc region of IgG antibodies and mediate humoral immune responses.
Afucosylated IgG antibodies (reduced fucose content) bind Fc-gamma receptors more efficiently. This leads to excessive production of pro-inflammatory cytokines and initiation of antibody-mediated cellular cytotoxicity.
In COVID-19 patients, SARS-CoV-2-mediated induction in afucosylated anti-spike IgG levels has been observed.
The majority of COVID-19 vaccines contain SARS-CoV-2 spike protein as an immunogen. Thus, it is possible that vaccine-induced spike expression in host cells could lead to afucosylated IgG response.
Based on this hypothesis, the scientists in the current study have assessed the impact of COVID-19 vaccination on anti-spike IgG glycosylation in individuals with or without prior SARS-CoV-2 exposure.
In infection-naïve individuals, about 25% of anti-spike IgG1 Fc were found to be afucosylated initially after administration of the first vaccine dose. However, the level decreased gradually with time.
In individuals with previous infection, about 2 – 10% of anti-spike IgG1 Fc were found to be afucosylated before vaccination.
Moreover, a significant correlation was observed between first dose-induced anti-spike afucosylation and second dose-induced anti-spike IgG antibodies.
[me: in other words a higher amount of afucosylation leads to more antibody production when given the 2nd dose and presumably if then infected]
[me: So while this study made no mention of monocytes it might be implied that the Afucosylated IgG antibodies might lead to more infected monocytes upon infection and more inflammation that is associated with more severe COVID
OK, so it seems shortly after vaccination, especially the first dose there are more antibodies being produced early that could cause mote serious disease. This could explain the bump in COVID deaths during the first roll outs of the vaccines in December 2020/January 2021
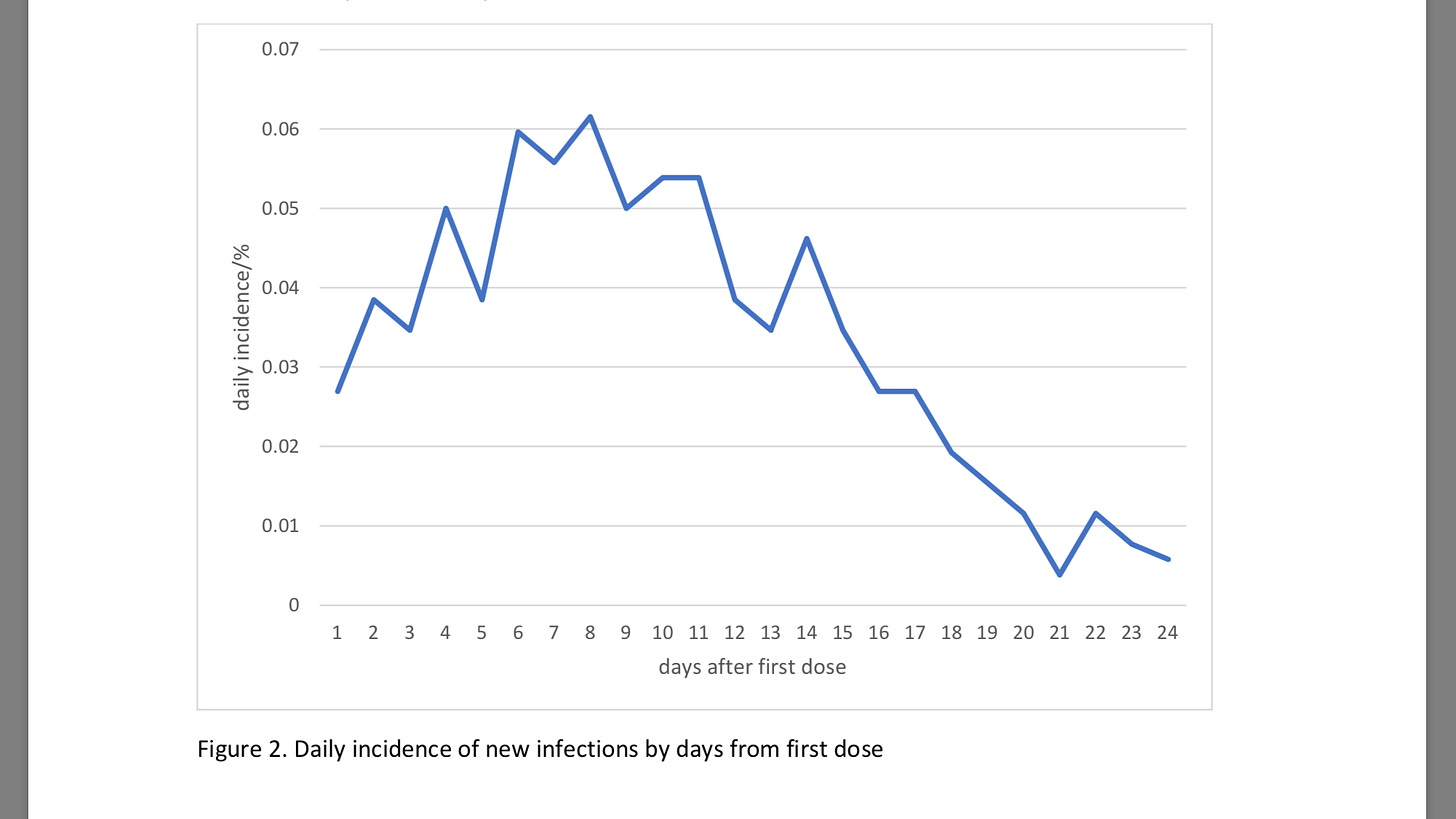
On top of that studies have shown infection rate rises within 14 days of the first dose
https://www.medrxiv.org/content/10.1101/2021.02.01.21250957v1.full.pdf
So you know, vaccinating the elderly with a first dose in the middle of a Pandemic when the prevalence of circulating virus is high might not be such a good idea]