New Paxlovid and IVM Studies
Recently a couple of new studies on IVM and Paxlovid have come out.
The IVM study was another study designed to fail. Treatment allowed late, sub-optimal dosage, short duration of treatment of 3 days) .
Paxlovid study showed effectiveness but less effective than Pfizers 88% claim (only 46%) and patients treated earlier and for 5 days.
IVM Study
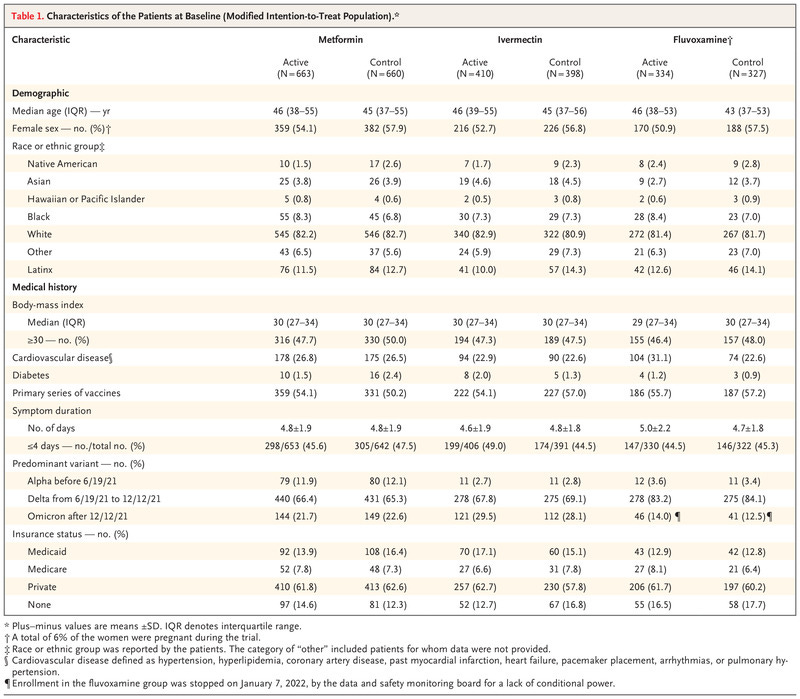
Eligibility criteria included an age of 30 to 85 years; a body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) associated with overweight or obesity; proof of SARS-CoV-2 infection within the past 3 days; and an onset of symptoms within 7 days before randomization
The exclusion criteria was vast and removed from the study many of those at highest risk. It also included those who were asymptomatic and thus unlikely to receive a benefit
The groups received the trial drugs according to the following doses: immediate-release metformin administered with an increase in dose over 6 days to 1500 mg per day for 14 days, ivermectin at a dose of 390 to 470 μg per kilogram per day for 3 days, and fluvoxamine at a dose of 50 mg twice daily for 14 days
[no other anti-viral is used for 3 days - molnupiravir and paxlovid are 5 days. ]
FLCC
Ivermectin: 0.3 to 0.6 mg/kg [300-600 μg per kilogram] one dose daily for at least 5 days or until symptoms resolve. If symptoms persist longer than 5 days, consult a healthcare provider….. For COVID treatment, ivermectin is best taken with a meal or just following a meal, for greater absorption.
https://covid19.onedaymd.com/2022/07/flccc-i-care-covid-treatment-protocol.html?m=1
Yet the study instructed
Route and Rate of Administration:
Ivermectin or matching placebo should be taken by mouth on an empty stomach with water. 1 hour before or 2 hours after a meal.
All other agents should be taken by mouth at the end of a balanced snack or small meal.
End Point
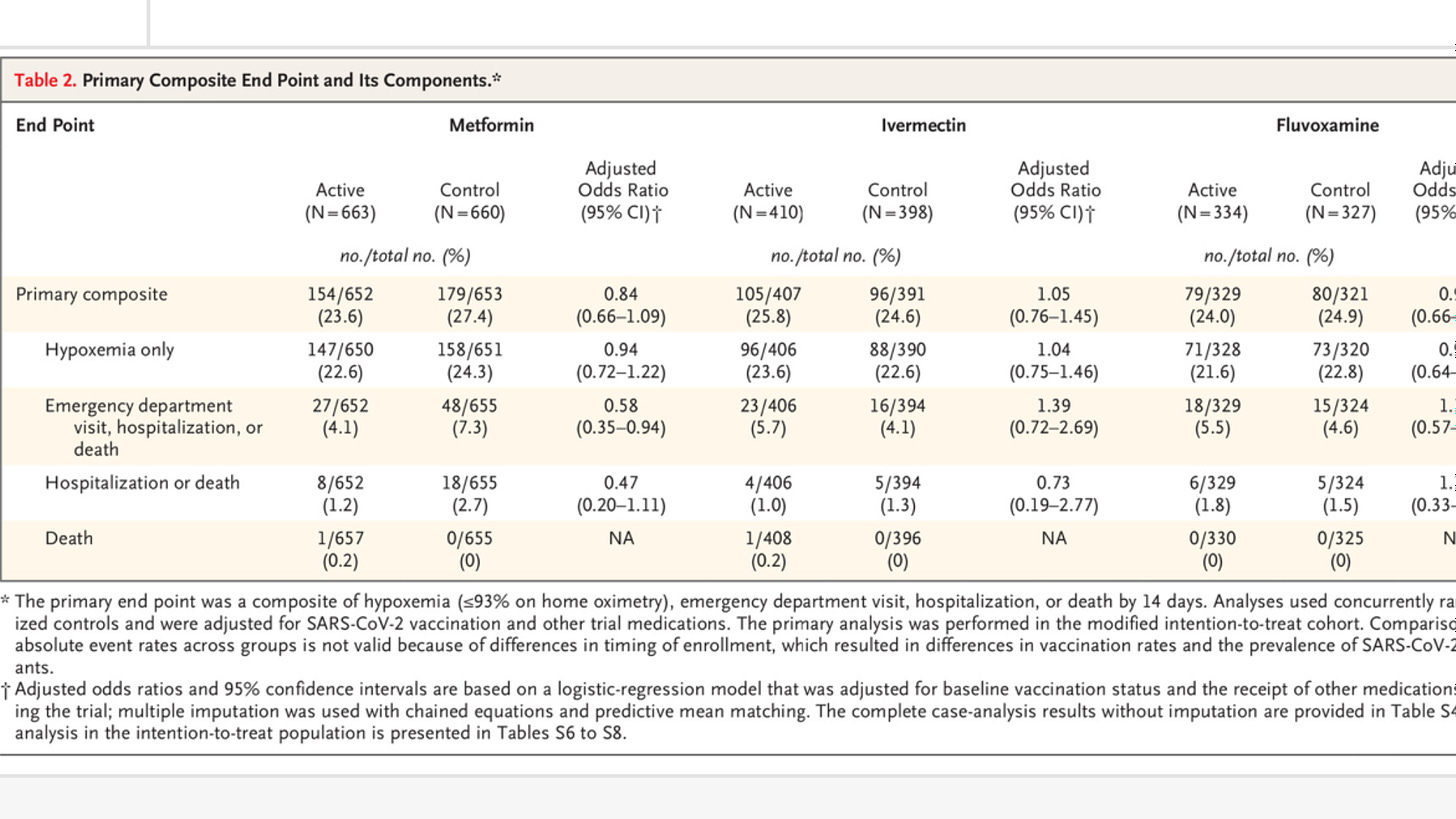
The primary end point was severe Covid-19 through 14 days, defined as a composite of hypoxemia (≤93% oxygen saturation on home oximetry), emergency department visit, hospitalization, or death
Results
Ta-da, OR is 0.73 for hospitalization or death. Too bad the study is too small to achieve statistical significance
More data from supplementary tables
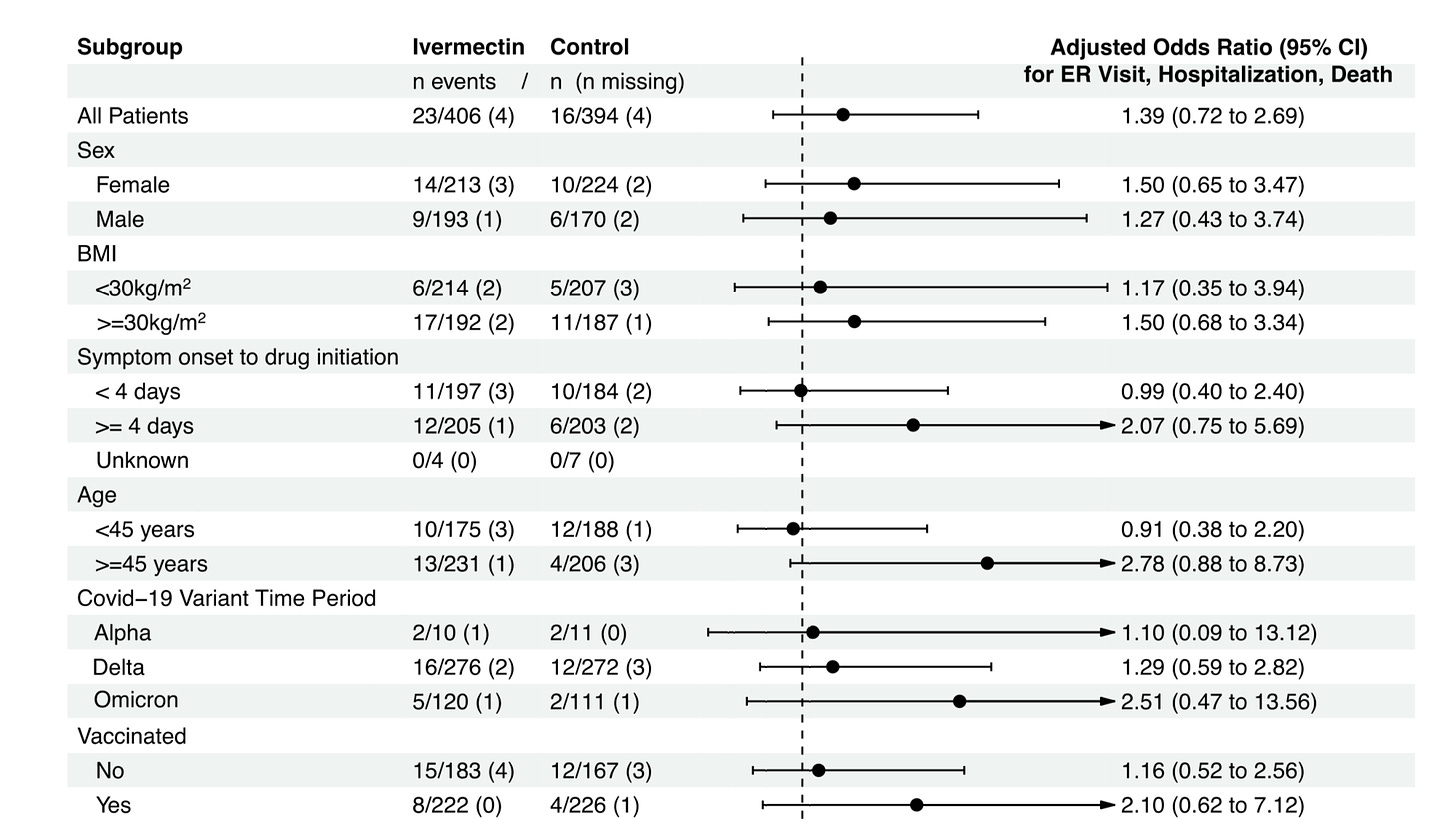
[giving IVM 4 or more days after symptoms develop is too late, and is the case for over 50% of those in the IVM arm . Clearly those treated earlier faired much better (0.99 for <4 days and 2.07 for 4-7 days) and IVM reduced ER visit or hospitalization, albeit marginally. The fact it was so marginal is because of the short duration of treatment (3 days) and sub-optimal dosage/administration]
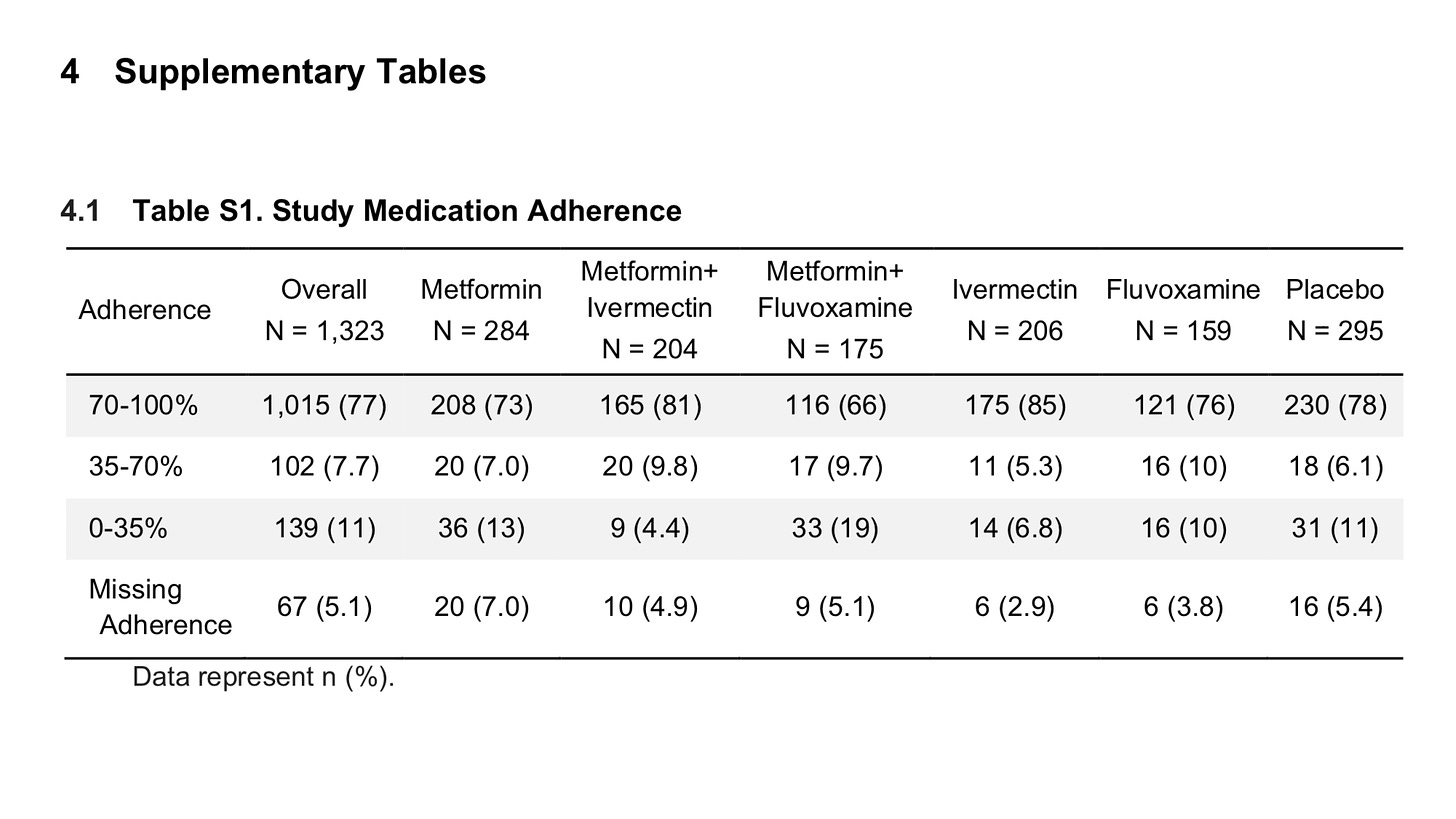
One interesting note is that only 66% of the IVM patients took all 3 doses
https://www.nejm.org/doi/full/10.1056/NEJMoa2201662?query=featured_home
Anyways, just another crap study designed to fail. NEJM has embarrassed itself in the COVID ERA (like Lancet)
Now lets look at the Paxlovid study out of Israel
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9214014/#!po=35.9649
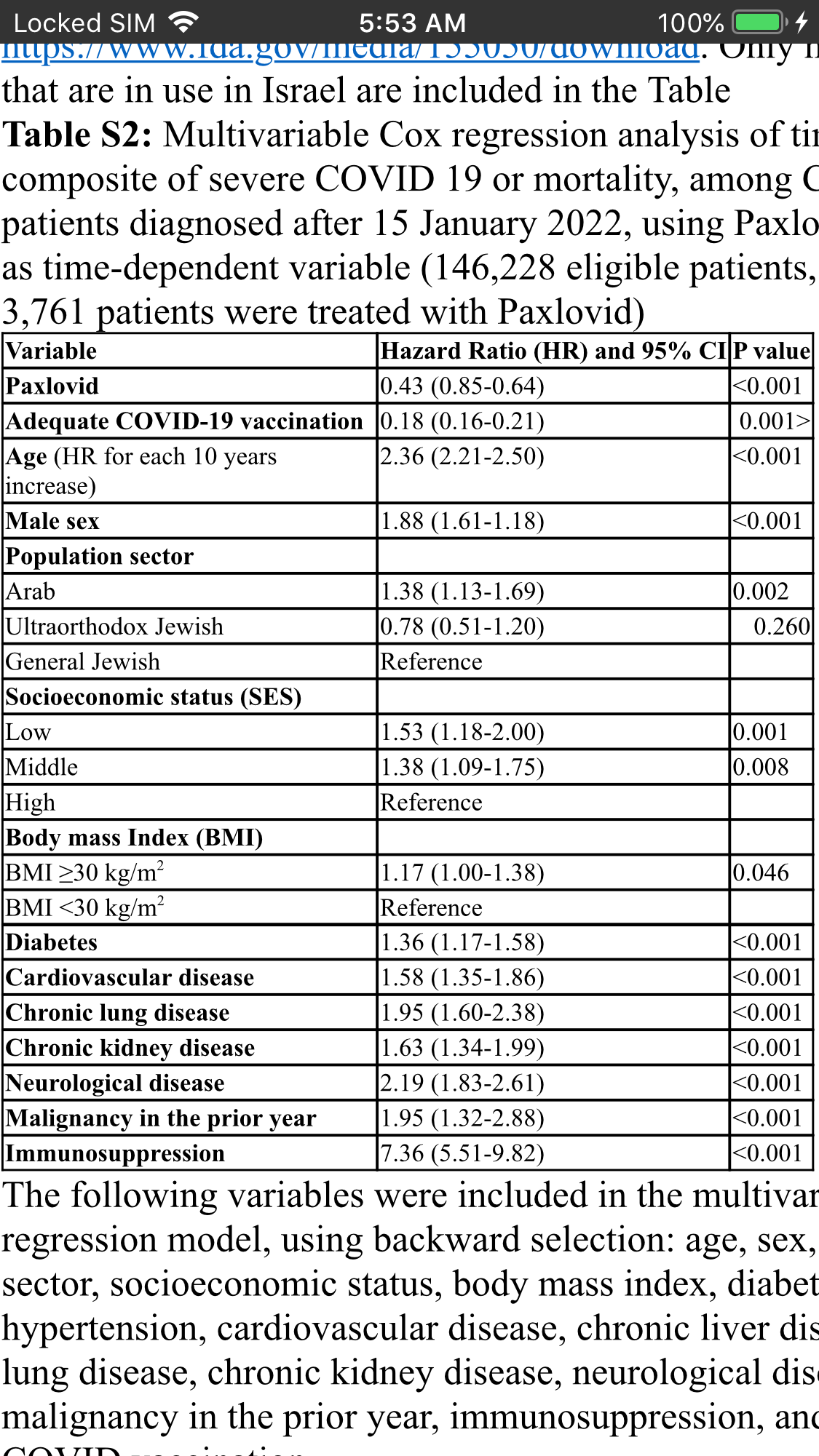
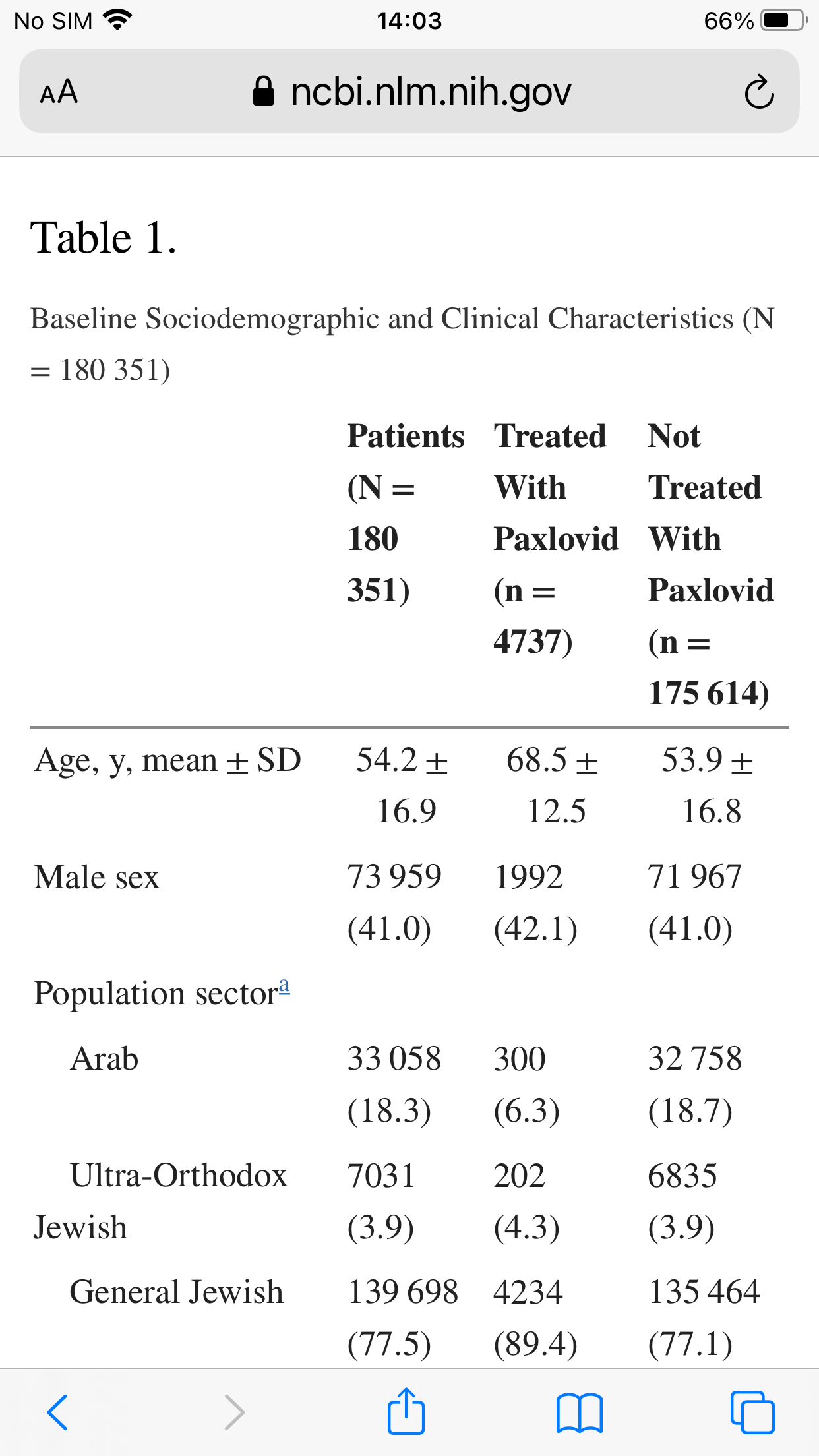
Overall, 180 351 eligible patients were included; of these, only 4737 (2.6%) were treated with Paxlovid, and 135 482 (75.1%) had adequate COVID-19 vaccination status. Both Paxlovid and adequate COVID-19 vaccination status were associated with significant decrease in the rate of severe COVID-19 or mortality with adjusted HRs of 0.54 (95% confidence interval [CI], .39–.75) and 0.20 (95% CI, .17–.22), respectively. Paxlovid appears to be more effective in older patients, immunosuppressed patients, and patients with underlying neurological or cardiovascular disease (interaction P < .05 for all
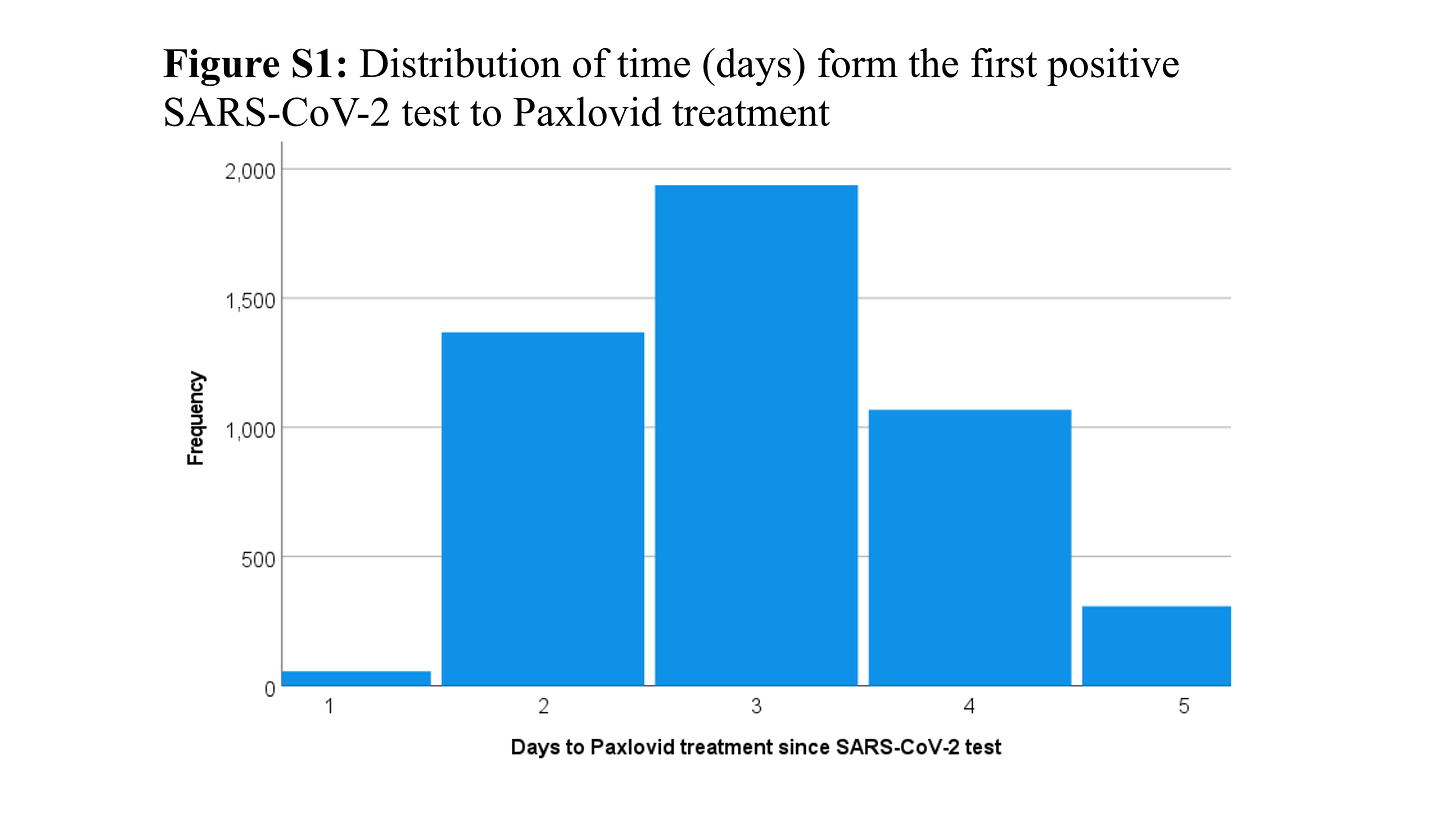
Overall, 4737 received Paxlovid; of these, 3361 (71.0%) received Paxlovid within 3 days of a positive test for SARS-CoV-2 infection
[In the IVM study above treatment could begin as late as 7 days after symptoms began - but within 3 days of a positive test]
Paxlovid is a new oral antiviral drug, produced by Pfizer for use against COVID-19, given for 5 consecutive days to patients with mild to moderate disease. Paxlovid consists of nirmatrelvir, a novel SARS-CoV-2 main protease inhibitor targeting 3CLpro of SARS-CoV-2, plus ritonavir (for its action as an inhibitor of cytochrome P450 3A4 to decrease nirmatrelvir metabolism and increase its serum levels)
[Unlike IVM (3 days) Paxlovid is given for 5 days]
A total of 39 events occurred in the 4737 patients treated with Paxlovid, reflecting a crude incidence rate of 10.4 per 1000 person-months, whereas 903 events occurred in the 175 614 patients not treated with Paxlovid, reflecting a crude incidence rate of 5.6 per 1000 person-months. The higher crude incidence rate in treated patients is likely attributed to older age and higher frequency of underlying comorbidities
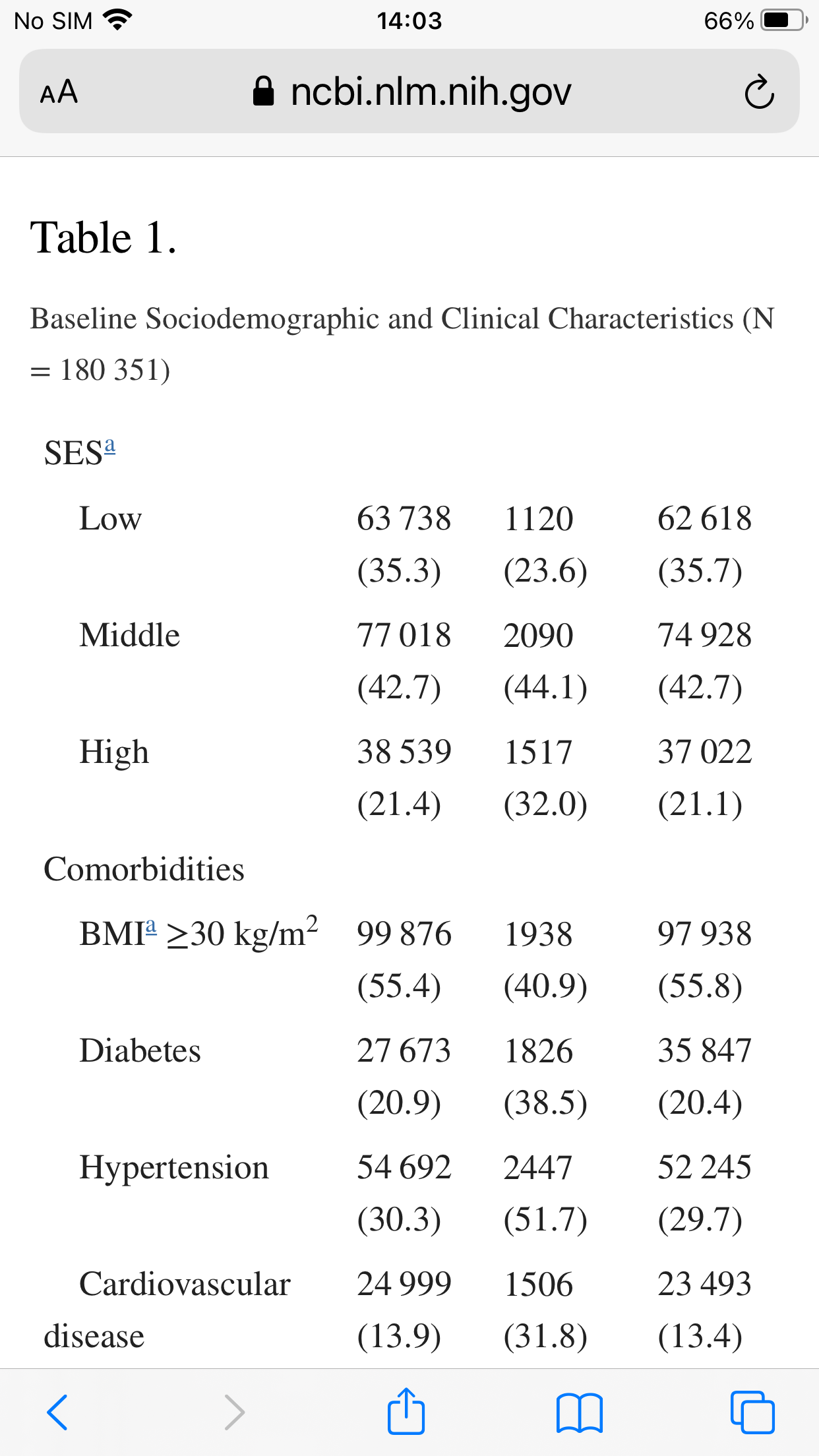
The mean age of eligible patients was 54.2 (SD, 16.9), 73 959 (41.0%) were males, and 135 482 (75.1%) had adequate COVID-19 vaccination status. Patients treated with Paxlovid were older, more likely to be male, more likely to belong to the Jewish population sector and to higher socioeconomic class, and in general more likely to have higher frequency of underlying comorbidities.
a
b
While the non-treatment group was younger it was also more obese
If you were poor and Arab you were less likely to get Paxlovid
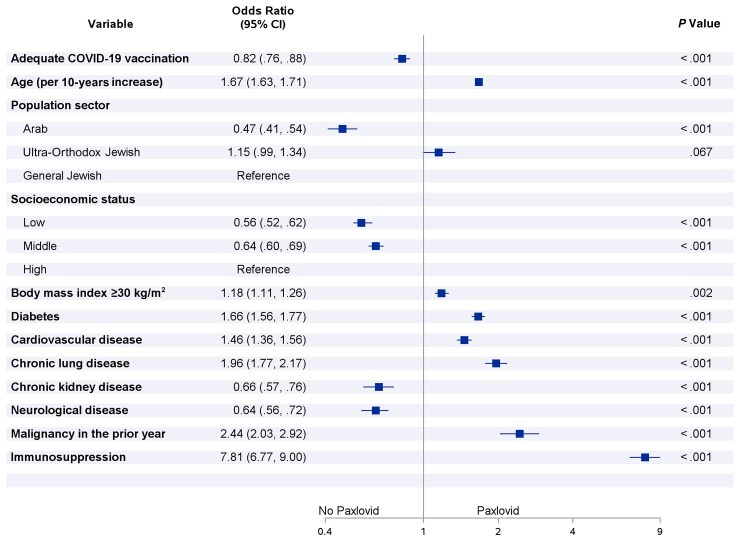
Multivariable odds ratios for risk factors associated with starting Paxlovid treatment within 5 days after positive test for severe acute respiratory syndrome coronavirus 2
Paxlovid was independently associated with a significantly decreased risk for the composite of severe COVID-19 or mortality, in a multivariable Cox regression models, with an HR of .54 (95% CI, .39–.75).
A sensitivity analysis, restricted to patients diagnosed with COVID-19 after mid-January, when the Omicron variant was the prevailing circulating variant, included 146 228 eligible patients, of whom 3761 were treated with Paxlovid. This analysis shows that Paxlovid was associated with greater decrease in the composite of severe COVID-19 and mortality (HR, 0.43 [95% CI, .85–.64])
The magnitude of Paxlovid effectiveness appears to be unrelated to COVID-19 vaccination status (P value for interaction = .129)
The magnitude of risk reduction was larger in the EPIC-HR trial (88%) than in the current study (46%). The lower risk reduction we have observed in the real-world setting might be explained by several differences between the studies, including those related to the virus, study design, and settings.
Moreover, Paxlovid might have been administered earlier in the trial compared to patients in this real-life cohort, as treatment in the trial was given up to 5 days from symptoms onset [in the IVM study above it was 7 days], while in the current study patients were included up to 5 days from SARS-CoV-2 laboratory confirmation; assuming that symptoms usually precede laboratory confirmation, higher effectiveness of earlier treatment is expected in the trial.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9214014/#!po=35.9649
Conclusion- Paxlovid is effective but not everyone can take it. Exclusion criteria were use of medications that were contraindicated for use with Paxlovid , estimated glomerular filtration rate <30 mL/minute/1.73 m2, dialysis, weight <40 kg, or pregnancy.
IVM is effective when used early at appropriate dosage and duration based on many clinicians and a large number of small RCT’s. However the high profile RCT’s like this one seem designed to fail
IVM is cheap , unlike Paxlovid which is about 700 USD per course). It has a clear safety profile being an approved drug in use for over 40 years (unlike Paxlovid which is EUA) and has few contraindications and drug interactions Paxlovid.
Yet guys like Alex Berenson keep pimping Paxlovid and trashing IVM (no wonder Twitter let him back on).