More on RSV
ICAN Letter to CDC raises good points
For 6 to 23-month-olds: “Within 28 days after vaccination, some respiratory tract-related infections were reported with greater frequency in the mRNA-1273 group compared to the placebo group, including croup, respiratory syncytial virus (RSV), and pneumonia. Events of croup were reported by 1.3% of mRNA-1273 recipients and 0.3% of placebo recipients, RSV by 0.8% of mRNA-1273 recipients and 0.5% of placebo recipients, and pneumonia by 0.2% of mRNA-1273 recipients and no placebo recipients.”
For 2 to 5-year-olds: “Within 28 days after vaccination, some respiratory tract-related infections were reported with greater frequency in the mRNA-1273 group than in the placebo group. Events of pneumonia were reported by 0.3% and 0% of mRNA- 1273 and placebo recipients, respectively. Respiratory syncytial virus (RSV) infection was reported by 0.4% and <0.1% of mRNA-1273 and placebo recipients, respectively.
• For 6 to 11-year-olds: “Within 28 days after vaccination, some respiratory tract infection-related PTs were reported more frequently in the vaccine group compared to the placebo group, such as Respiratory syncytial virus infection (0.3% vs 0%) and Upper respiratory tract infection (3.9% vs 2.5%). An analysis including all respiratory-tract infection related PTs, except COVID-19, showed a small imbalance of 5.9% in the vaccine group compared to 4.4% in the placebo group.”
Here is the Moderna study.
https://www.fda.gov/media/159611/download
Pfizers vaccine causes a similar effect, which is known as viral interference.
In the clinical trial for Pfizer’s pediatric Covid-19 vaccine (BNT162b2), serious adverse events “reported in the BNT162b2 group included RSV bronchiolitis (5 participants), pneumonia (2 participants), gastroenteritis (2 participants), lower respiratory tract infection (2 participants).”
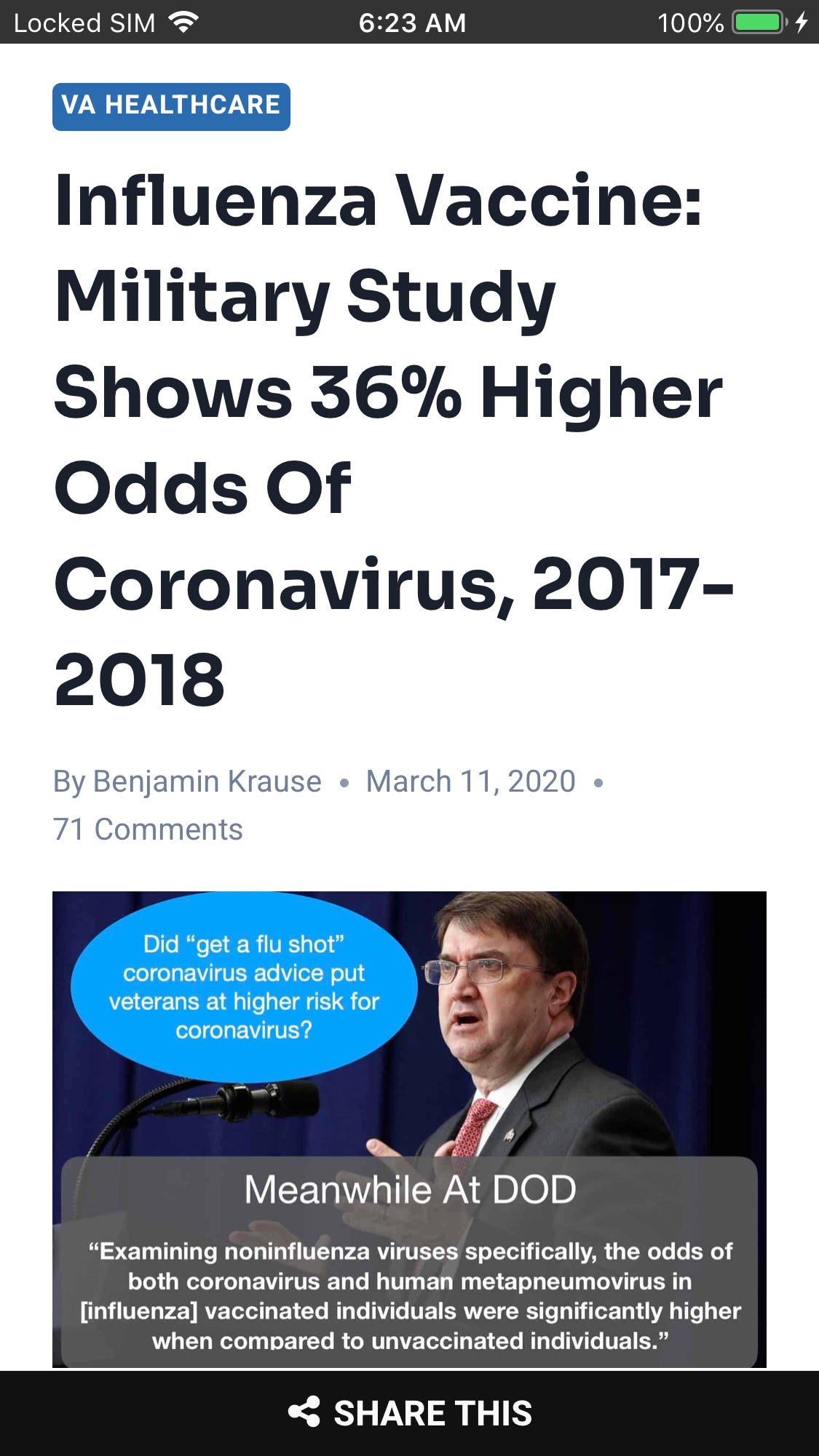
Viral interference can either cause a reduction in infection from other infections or an increase in infections. For example a DoD study showed flu vaccination can result in increased coronavirus infections (before Sars-Cov-2)
https://www.disabledveterans.org/2020/03/11/flu-vaccine-increases-coronavirus-risk/
Some people speculate the reduction in flu since COVID is due to viral interference of Sars-Cov-2 reducing influenza virus transmission
So scanning the news headlines on RSV outbreaks
1/
2/
3/
I wonder what the Childhood COVID vaccination rates in Massachusetts, Michigan, and New York ? And why isn’t anyone entertaining the possibility of COVID VACCINATION being related to these unusual outbreaks
RSV was only first discovered sometime after the Polio Vaccine was developed. Some speculate it was due to lab release from infected monkeys used to provide kidneys needed to culture the polio virus for the vaccines. I mentioned this in my last post.
There are also some RSV Vaccine trials underway. Some speculate an Emergency may be declared (hence they MSM hype) which will allow them to fast track the vaccines at Warp Speed like the COVID Vaccines.
Modernas mRNA vax is targeting Children and would be the biggest beneficiary of a Emergency Declaration.
Pfizer has a traditional vaccine for pregnant women in Phase III Trials and may be near approval
The pre-planned, interim efficacy analysis conducted by an external and independent Data Monitoring Committee (DMC) met the success criterion for one of two primary endpoints. The observed efficacy for severe medically attended lower respiratory tract illness (severe MA-LRTI) was 81.8% (CI: 40.6%, 96.3%) through the first 90 days of life. Substantial efficacy of 69.4% (CI: 44.3%, 84.1%) was demonstrated for infants over the six-month follow-up period.
Although the statistical success criterion was not met for the second primary endpoint, clinically meaningful efficacy was observed for MA-LRTI of 57.1% (CI: 14.7%, 79.8%) in infants from birth through the first 90 days of life. Efficacy for MA-LRTI of 51.3% (CI: 29.4%, 66.8%) was observed over the six-month follow up period.
The study enrolled approximately 7,400 pregnant individuals. Maternal participants ≤ 49 years of age were randomized in a 1:1 ratio to receive a single dose of either 120 µg of Pfizer’s RSVpreF or placebo during the late second to third trimester of their pregnancy. The trial also assessed safety throughout the study and immunogenicity of the vaccine in pregnant individuals and their infants. Maternal participants were followed for safety through vaccination and for six months after delivery. Infants were followed for at least one year for safety and efficacy, with over half of the infants followed for two years. This was a global study in 18 countries and started in June 2020, so it spanned multiple RSV seasons in both the northern and southern hemisphere.
Pfizer intends to submit these results for peer-review in a scientific journal.
On March 2, 2022, Pfizer announced that its vaccine candidate received Breakthrough Therapy Designation from the FDA for the prevention of RSV-associated lower respiratory tract disease in infants up to six months of age by active immunization of pregnant women.
In the United States alone, approximately 2.1 million outpatient visits and 58,000 hospitalizations due to RSV occur each year among children younger than five years old.
[Me- there are almost 20 million children 0-5, so they are saying 1/333 are hospitalized each year for RSV. Putting that into perspective, in a study of 7400, one would expect 5-6 kids to be hospitalized within 90 days (severe medically attended). The trial size is way too undersized IMO]
Worldwide, RSV results in death of approximately 102,000 children annually, with about half of those in infants less than 6 months old and the vast majority in developing countries.8,9
RSV bronchiolitis is the leading cause of infant hospitalization due to viral respiratory illness, characterized by respiratory distress that can result in death. There is no specific treatment for RSV, only supportive care measures like oxygen and fluids. Currently there is no vaccine to prevent RSV.
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-announces-positive-top-line-data-phase-3-global
Wow, 1 -2 year follow up on the kids. Lol. That means it is stopped before anyone can dx autism or other developmental issues
Also, note that they say no treatment available, so clearly a vaccine is needed.
Oh wait.
Look at this. Human RSV Vaccine Candidates work to prevent Baby Cows from getting RSV
That means what works for humans RSV works for cows RSV and maybe vice versa
And looky here (h/t Highwire)
..
Anyone starting to get the idea IVM is this virus killer (and vaccine killer) wonder drug that must keep Vaccine Profitologists up at night? Their worst nightmare.
I can see FDA next IVM campaign coming. YOUR CHILD IS NOT A COW
Ya think anyone is thinking of doing an IVM trial on these poor kids in hospital with RSV following their COVID SHOTS (at least, some of them got it) ? I doubt it.
I am starting to think the Medical Profession and Society at large are under a Satanic Demons possession