IgG4 Abs, Tregs, Afucosylated Antibodies -Oh My!
A couple of papers have come out recently and generated a few substack articles already. Igor and Berenson have been on top of this (you know them).
The papers touch on Immune Tolerance caused after repeated boosting by IgG4, increased T-Tegs (in mice after repeated boosting with a non-mRNA vaccine), and possible Antibody Enhancement to those naive to COVID getting a 1st dose of mRNA due to afucosylated IgG1 antibodies if infected shortly after vaccination
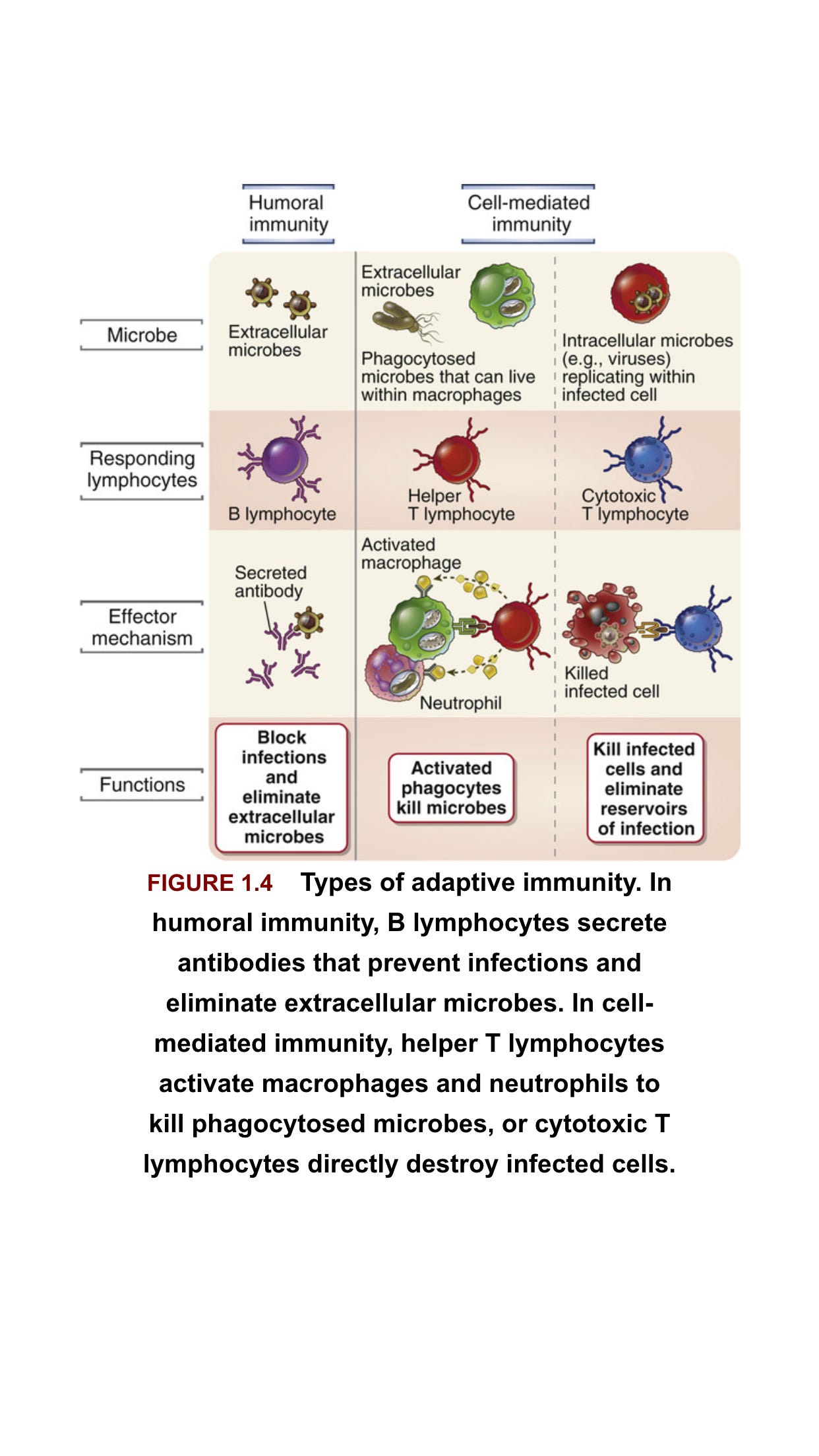
So this post will have 2 sections. The first focusing on the Humoral (Antibody) Immunity and the second on the Cellular Immunity (T-Cells), these are the two parts of the Adoptive Immune System which backs up the front line Immune Defense known as the Innate Immune System
Too long for Email so click on Title to see the whole thing
I-Humoral (Antibody) Immunity
Most have focused on IgA4 and I will talk about that too, along with IgA and Afucosylated Antibodies and Antibodies in general
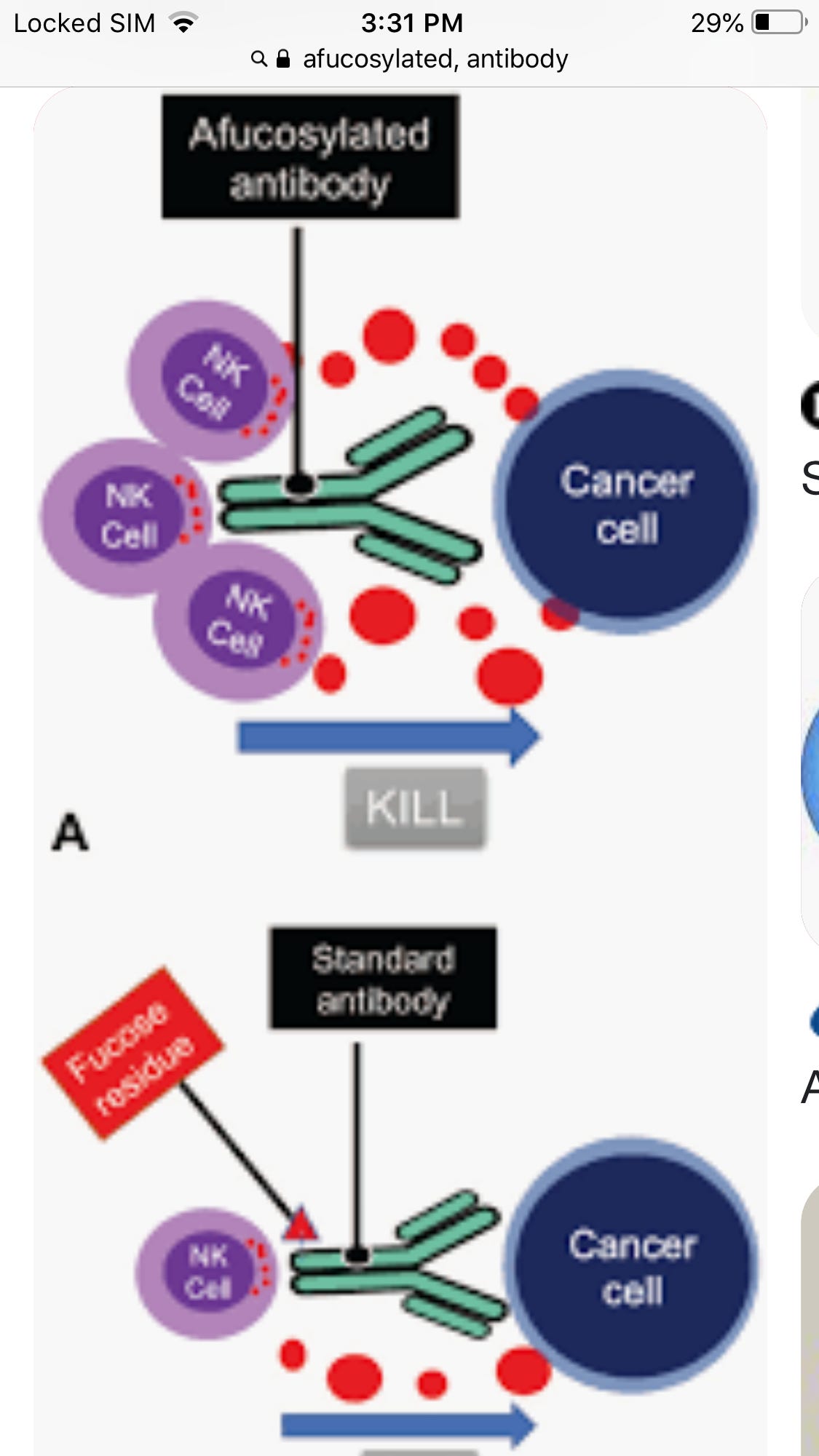
Afucosylated Antibodies
Turns out those people who get real sick with COVID are getting real sick in part due to bad antibodies. These IgG are afucosylated. A FOOKS AH WHAT?
Bad is a relative term though. If you have cancer afucosylated antibodies are good. They increase cellular destruction
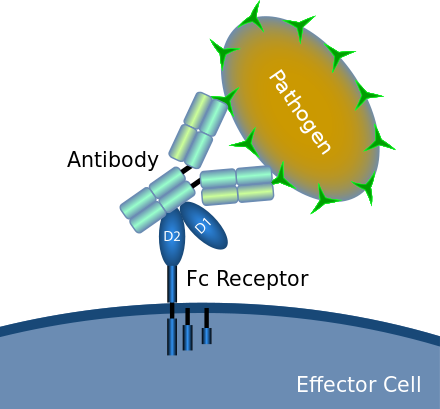
Without getting too deep into the weeds the straight part is what hooks up with immune cells after the two arms latch on to an antigen like one a cancer cell. It binds with the immune cells and gives instructions. In this case come all, come many, kill,kill, kill.
But this can cause problems if it gets out of hands like it can with COVID. Its not the virus making the patient sick its their immune system going bonkers
Afucosylated antibodies are part of that. Typically, its transient and drops to safe levels after awhile, so if you aren’t infected or if you are but the Afucosylated abs aren’t too many, you do ok. The problem is, if you never were infected before and take an mRNA shot you make a lot of Afucosylated abs for a bit. Thats a problem if you get infected shortly after the first dose, and we know from many studies that you are more likely to get infected after a 1st dose due to transient immune suppression
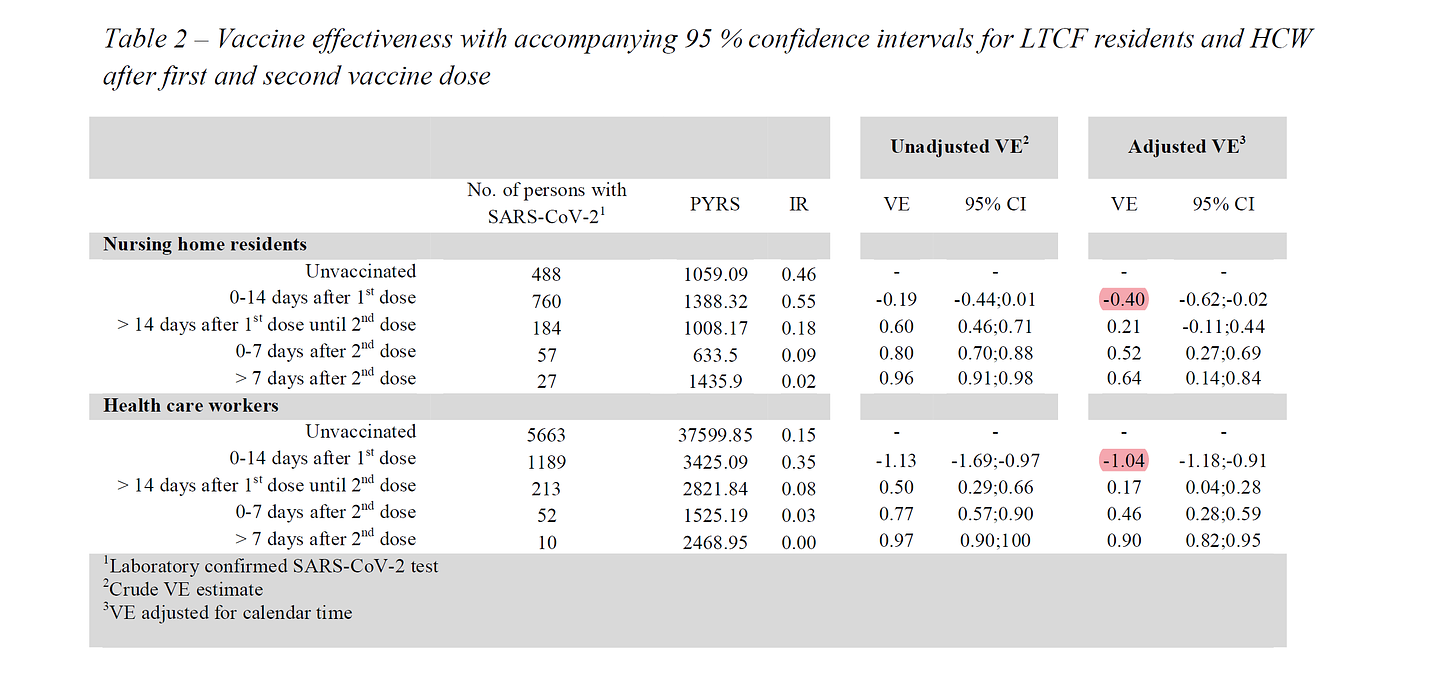
in this STUDY the danes found dramatic increases in infection rates post vaccination. “adjusted VE” is the risk adjusted vaccine efficacy. it was -40% for nursing home residents and -104% for health care workers in those homes. it more than doubled their risk. (it’s to be expected that the younger, healthier HCW’s would see more drop vs the NH residents in the event of immuno-suppression as they had more effective immune systems to suppress. we see the same reflected in peak VE of 90% for HCW vs 64% in residents)
note that this is all in the 14 days post dose VE ramped up over time, but those first two weeks were a serious risk accelerant. 40-100% rise in risk is no joke.
https://www.medrxiv.org/content/10.1101/2021.03.08.21252200v1
and this study does not stand alone. it was, in fact, validated in pfizer’s own data as laid out here by dr clare craig, a UK pathologist
“Indeed, the original Pfizer trial demonstrated a statistically significant 40% increase in ‘suspected COVID’, with 409 cases in the vaccination arm in the first week of the trial, compared with 287 in the placebo arm. Other publications have omitted mention of the period immediately after vaccination. There is substantial anecdotal evidence of people who had tested negative prior to vaccination, becoming infected shortly afterwards, invariably attributed to exposure just before vaccination.Others have raised concerns about this.”
https://www.bmj.com/content/372/bmj.n783/rr
More on Afucosylated IgG1 after vaccination in COVID naive in a recent study to follow below.
IgG4 and Vaccination
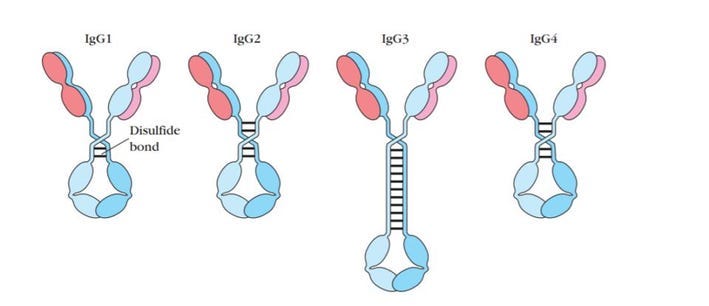
The constant regions of IgG Antibodies for all four of these subclasses are derived from combinations of germline inherited DNA sequences, which are serially constructed.
The rarest among these are IgG4 antibodies. All four antibody subclasses may bind to the same epitope, but they differ in the consequence of that binding.
The Fc portion (the trunk of the ‘Y’ shape) determines how the body reacts to an antigen once it is bound to the antibody. These reactions are effector functions, and each subclass activates effector functions to varying degrees.
The three main functions are 1) the activation of inflammation; (2) the opsonization (labeling) of pathogens and cells for clearance/destruction; (3) the direct killing of target cells/microbes by lysis.
IgG1, IgG2, and IgG3 provide protection not only by blocking the virus from entering cells but also by their Fc regions activating effector functions and signaling the immune system to kill infected cells. IgG4, however, does not activate the effector functions, meaning their presence may impact how the body responds to Covid-19.
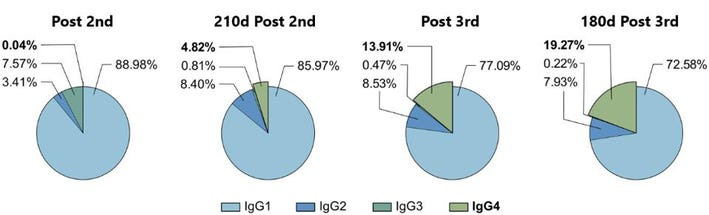
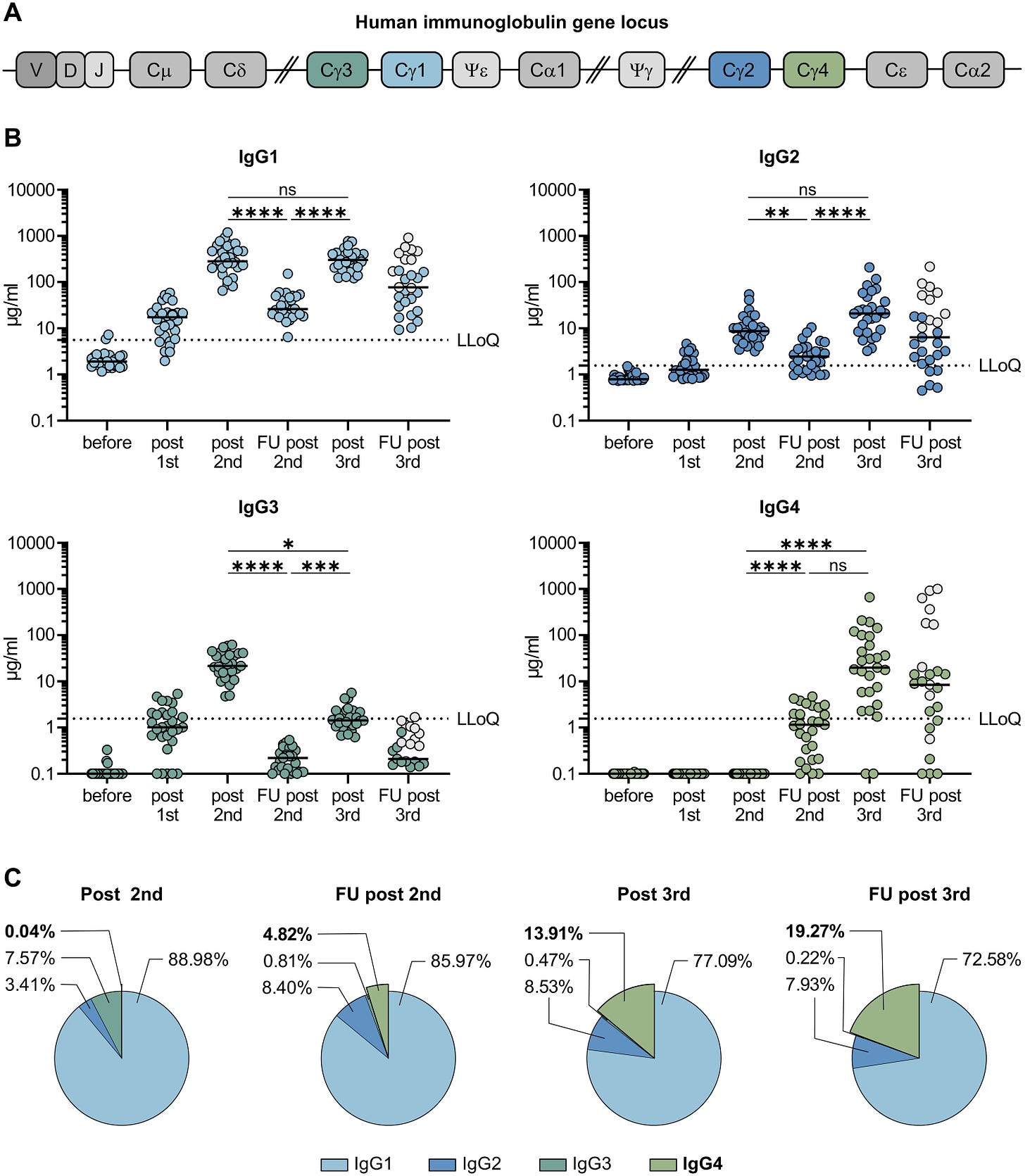
To measure the relative frequency of IgG subclasses in the sera of vaccine recipients, Irrgang et al. followed a cohort of 29 healthcare workers, analyzing their sera ten days after a first, second, and third dose, as well as 210 days after the second, and 180 days after the third.
In line with initial efficacy reports for the vaccine, antibody levels were robust throughout the cohort post-first and second doses. The researchers also found that 210 days after the second dose, antibody levels had fallen significantly, reaffirming the loss of antibody protection over time. Again, following the third dose, antibodies rose significantly, only to fall 180 days after the booster.
The most interesting data regards the growing concentration of IgG4 in the cohort’s sera. On average, only 0.04% of the antibody response post-second vaccination was IgG4. 210 days after the second dose, that percentage rose to 4.82%. Following the third dose, IgG4 comprised 13.91%, rising to 19.27% 180 days after.
Why is this happening? IgG4 antibodies have distinct characteristics. They are highly matured, highly variable, and have a high affinity for the receptor for the target. Therefore, they should be exceedingly effective at stopping the virus via neutralization activity and ACE2 direct blocking. Because it is not triggering the complement effector functions, IgG4’s sole and primary activity is blocking and neutralizing the virus.
The exact impacts of an increased frequency of IgG4 are unclear a could be argued either way as a positive or negative. IgG4 occurrence correlates with increased avidity but decreased antibody effector function. Avidity measures the overall or accumulated strength of a protein-protein complex, which may serve as a marker for increased virus neutralization. However, IgG4’s decreased effector functions could signal less robust neutralization and clearance of virus and infected cells.
The addition of IgG4 may explain the increased ability of sera from multiply vaccinated or infected patients to recognize a broader range of strains because it binds more tightly. While it lacks the effector functions, that may not be as serious a disadvantage as one may think. More than 80% of antibodies in analyzed sera were still IgG1, IgG2, and IgG3, meaning effector functions are still activated, creating a healthy mix. However, it remains to be seen how IgG4 concentrations impact disease outcomes such as hospitalization and death.
Lets look at that study paper a bit closer
Class switch towards non-inflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination
Dec 22, 2022
https://www.science.org/doi/10.1126/sciimmunol.ade2798
IgG4 antibodies among all spike-specific IgG antibodies rose on average from 0.04% shortly after the second vaccination to 19.27% late after the third vaccination
Importantly, this class switch was associated with a reduced capacity of the spike-specific antibodies to mediate antibody-dependent cellular phagocytosis and complement deposition.
It was further shown that antibody avidity increased following mRNA booster vaccination, which was partly explained by prolonged germinal center (GC) activation and ongoing B-cell maturation. SARS-CoV-2 vaccine-derived mRNA and spike protein was detected even several weeks after vaccination.
Sequencing of memory B cells revealed somatic hypermutation (SHM) in GCs for up to six months, which resulted in broadening and diversification of the memory B cell repertoire as well as in an improved effectiveness against VOC
Activation-induced cytidine deaminase (AID) is the enzyme that catalyzes SHM in antibody variable (V) regions. It is expressed in GC B cells and also mediates class switch recombination (CSR) of constant (C) region genes .
Ongoing activity of AID can lead to switching towards more downstream Cγ regions, i.e. γ1, γ2 and γ4, which encode IgG1, IgG2 and IgG4
However, the regulators for germline transcription of the γ2 and γ4 gene locus are not very well understood in humans. IL-4 in concert with IL-10 has been described to be involved in switching to IgG4
[as we will see in the mice study below IL-10 is increased after repeated boosting along with increase in T-Regs which serves to down-regulate the cellular response and may play some role in doing the same for the humoral-antibody response]
IgG2 and IgG4, were reported to mediate mostly non-inflammatory or even anti-inflammatory functions due to decreased Fc-mediated antibody effector functions including antibody-dependent cellular phagocytosis (ADCP), cellular cytotoxicity (ADCC) and complement deposition (ADCD)
While we confirmed an increased antibody avidity and higher neutralization capacity against the recently emerged Omicron VOC after the third vaccine dose, the switch towards distal IgG subclasses was accompanied by reduced fragment crystallizable (Fc) gamma receptor (FcγR)-mediated effector functions such as ADCP and ADCD.
Intriguingly, 210 days after the second immunization, the levels of spike-specific IgG4 antibodies exceeded the lower limit of quantification in the sera of about half of the vaccinees. The levels for all other subclasses dropped significantly as expected from the overall anti-S response.
After the third mRNA immunization, the amounts of all IgG subclasses were elevated again and reached levels as measured shortly after the second vaccination in the case of IgG1 and IgG2 . IgG3 remained at lower levels compared to the time point shortly after second vaccination. Notably, a marked increase in IgG4 antibody levels was observed after the booster immunization in nearly all vaccinees. Until this time point, none of the participants reported an episode of SARS-CoV-2 infection and we could also not detect anti-nucleoprotein antibodies in any of the serum samples.
In four individuals IgG4 even became the most prominent IgG subclass after the third immunization. Specifically in individuals having experienced a [breakthrough] infection, IgG4 antibodies accounted for 40–80% of all anti-S antibodies
While the frequency of IgG4-expressing memory cells among non-spike binding memory B cells was in the range of 1–8% as described before , a significantly higher frequency of spike-binding memory B cells expressed IgG4 at all time points, reaching up to 37% of all IgG subclasses
Since the total amount of anti-spike IgG antibodies was only moderately (1.6-fold) elevated after the third compared to the second vaccine dose, we next investigated whether the increased proportion of IgG4 antibodies had functional consequences.
The avidity was clearly increased after the third vaccination , which is in line with recent reports .Furthermore, the capacities to bind trimeric spike protein and to prevent soluble RBD binding to ACE2 , which both serve as surrogate markers for virus neutralization, were increased after the third dose
IgG4 results in reduced activation of the FcγRIIA, which was reported to be a key mediator of ADCP . Consistent with this, sera taken after the third vaccination and normalized to the amount of anti-spike antibodies yielded significant lower phagocytic scores than sera from the same donors after two immunizations . Furthermore, antibody-dependent complement deposition on spike-coated microbeads was also significantly reduced after incubation with sera taken after the third vaccination. Together, these data show that spike protein-reactive IgG4 exhibit reduced Fc-mediated effector functions.
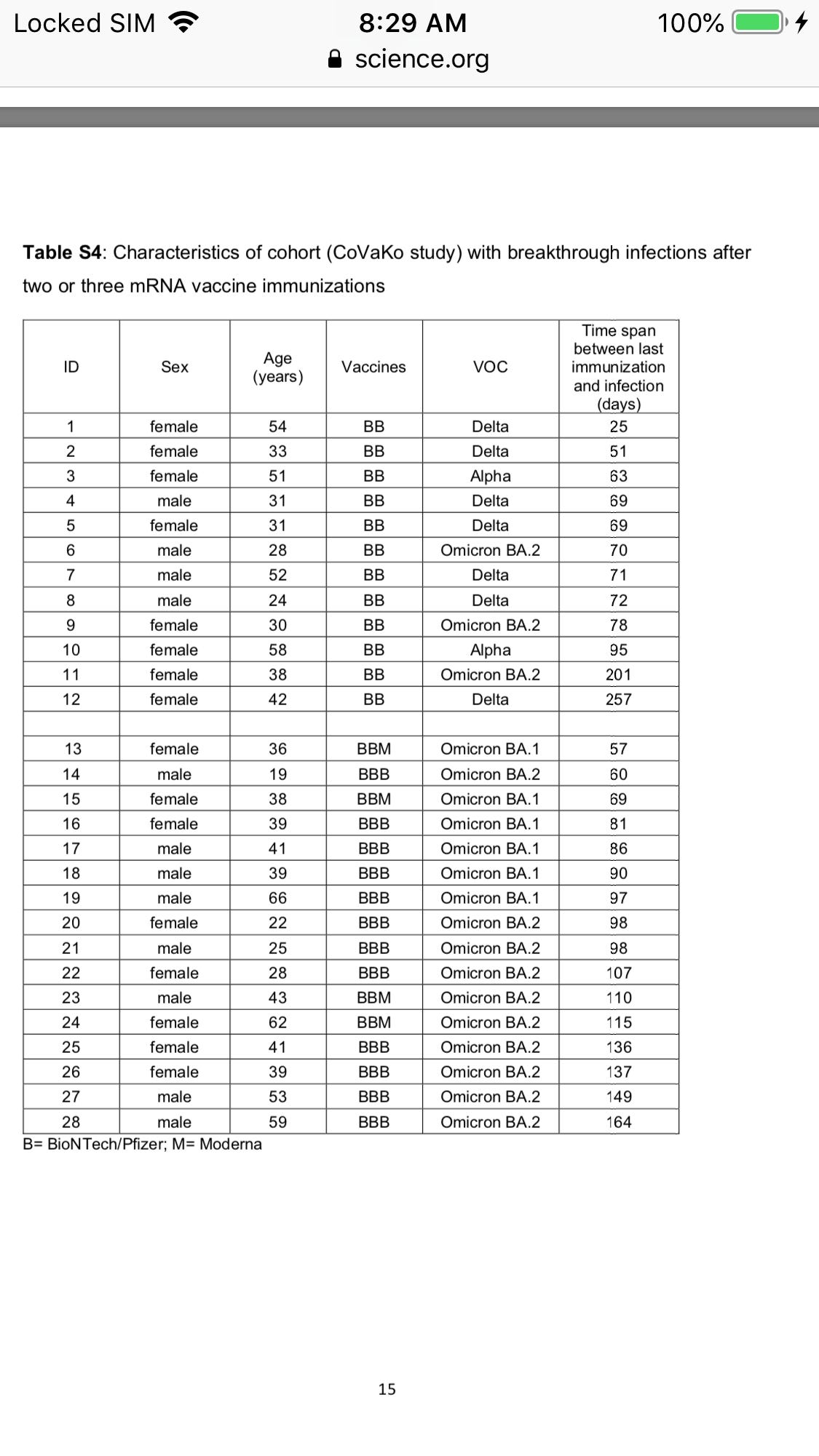
Interestingly, in the cohort that had two mRNA vaccinations before breakthrough infections, only three individuals developed IgG4 antibodies that were above the lower limit of quantification after infection. These three individuals experienced the infection with the largest time difference to the last vaccination, at 95, 201 or 257 days after the second vaccination, while in the other nine patients (without IgG4 after infection that was above the lower limit of quantification) the infection took place between 25 and 78 days after the second mRNA shot.
[12 of 37 in this cohort developed breakthrough infection after the 2nd dose from 25-257 days ]
Individuals having experienced a breakthrough infection within the first 70 days after the second vaccination did not have substantial serum levels of anti-spike IgG4 at their first visit, which also did not significantly increase during the following observation period. In contrast, anamnestic IgG4 responses were seen when breakthrough infections occurred later than 3 months after the second immunization, and were robustly detectable when the study participants had been vaccinated three times before infection
It is currently not clear whether or to what extent the Comirnaty mRNA vaccination or the short interval of immunizations are responsible for the observed long-lasting GC reactions , but a prolonged presence of vaccine mRNA or antigen in the lymph node might be a potential explanation . Furthermore, a robust and persistent T follicular helper cell (TFH) response for up to six months after mRNA vaccination has been described in draining lymph nodes , which might be involved in the regulation of CSR by recurrent interactions of GC B cells with TFH cells. Of note, our study was restricted to vaccinees receiving the Comirnaty vaccine
Indeed, in the present study we confirmed previous reports on improved avidity and neutralizing potential of vaccine-induced antibodies after the third vaccination . However, the large number of breakthrough infections caused by the Omicron variant indicates that current vaccination regimens do not confer sterilizing protection.
Once infection is established, Fc-mediated effector functions become more relevant to clear viral infections. Systemic serology approaches have even revealed that different antibody functions can contribute to various degrees to protection dependent on the viral pathogen, as shown for influenza viruses, RSV or SARS-CoV-2 . Passive immunization studies in animal models have further demonstrated that the degree of protection achieved by the application of monoclonal antibodies depends on their IgG subclass . In this regard, IgG4 is considered as an anti-inflammatory IgG with low potential to mediate Fc-dependent effector function such as ADCC or ADCP .
So far, only few studies on the role of vaccine-induced IgG4 responses against infectious diseases are available. In the field of HIV vaccine development, repeated protein immunization in the trial VAX003 led to higher levels of HIV gp120-specific IgG2 and IgG4
With respect to the control of viral infections, little is known regarding virus-specific IgG4 antibody responses. As shown here for RSV-specific IgG responses, IgG4 is hardly induced by acute respiratory viral infections even after repeated exposure. Although measles-specific IgG4 antibodies can be induced by natural infection , even chronic viral infections like HCMV do not trigger significant specific IgG4 antibodies .
There are very few reports on the induction of IgG4 after natural infection with SARS-CoV-2. The dominant subclasses were mostly IgG1 and IgG3 . Nevertheless, a Brazilian study during the early phase of the pandemic correlated an early onset and high levels of anti-spike IgG4 antibodies with a more severe COVID-19 progression after SARS-CoV-2 infection, which might indicate a less effective antibody response. Additionally, Della-Torre et al. reported on a significant association of high IgG4/IgG1 ratios with poor disease outcome . However, in the case of a primary immune response, the causality is difficult to address, since it is also possible that a more severe infection leads to an IgG4 response and not vice versa.
[yes, repeated COVID vaccination MIMICS a severe infection]
In our study, antibody-mediated phagocytic activity and complement deposition were reduced in sera after the third immunization, in parallel to higher proportions of anti-spike IgG4 antibodies. However, how these changes affect subsequent virus infections remains unclear. Since Fc-mediated effector function could be critical for viral clearance, an increase in IgG4 subclasses might result in longer viral persistence in case of infection. However, it is also conceivable that non-inflammatory Fc-mediated effector functions reduce immunopathology while virus is still being neutralized via high-avidity antibody variable regions.
In a cohort of vaccinees with breakthrough infections, we did not obtain any evidence for an alteration of disease severity, which was mild in almost all of our cases
[my thoughts. During early infection the battle is being fought in the respiratory mucosa. Serum IgG including IgG4 is transferred to the mucosa where its higher avidity is more beneficial to neutralization, and its anti-inflammatory properties reduces symptoms in the respiratory tract and may inhibit a later cytokine storm]
From the supplementary tables we see a total of 28 breakthrough infections (12 after the 2nd dose, 16 after the 3rd dose. The 2 cohorts include 66 participants so its a rather high breakthrough rate as 42% of the trial participants developed a breakthrough infection after 2 or 3 doses
The above study was followed by the study below. This study distinguished between those who were pre infected before vaccination and those who were naive. It also only looked at igG4 after 2 doses
mRNA vaccines against SARS-CoV-2 induce comparably low long-term IgG Fc galactosylation and sialylation levels but increasing long-term IgG4 responses compared to an adenovirus-based vaccine
Jan 12,2023
Before we get into it we needs to brush up on some terms
IgGs are glycoproteins and their glycosylation pattern can change during time, due to age, diseases or environmental factors
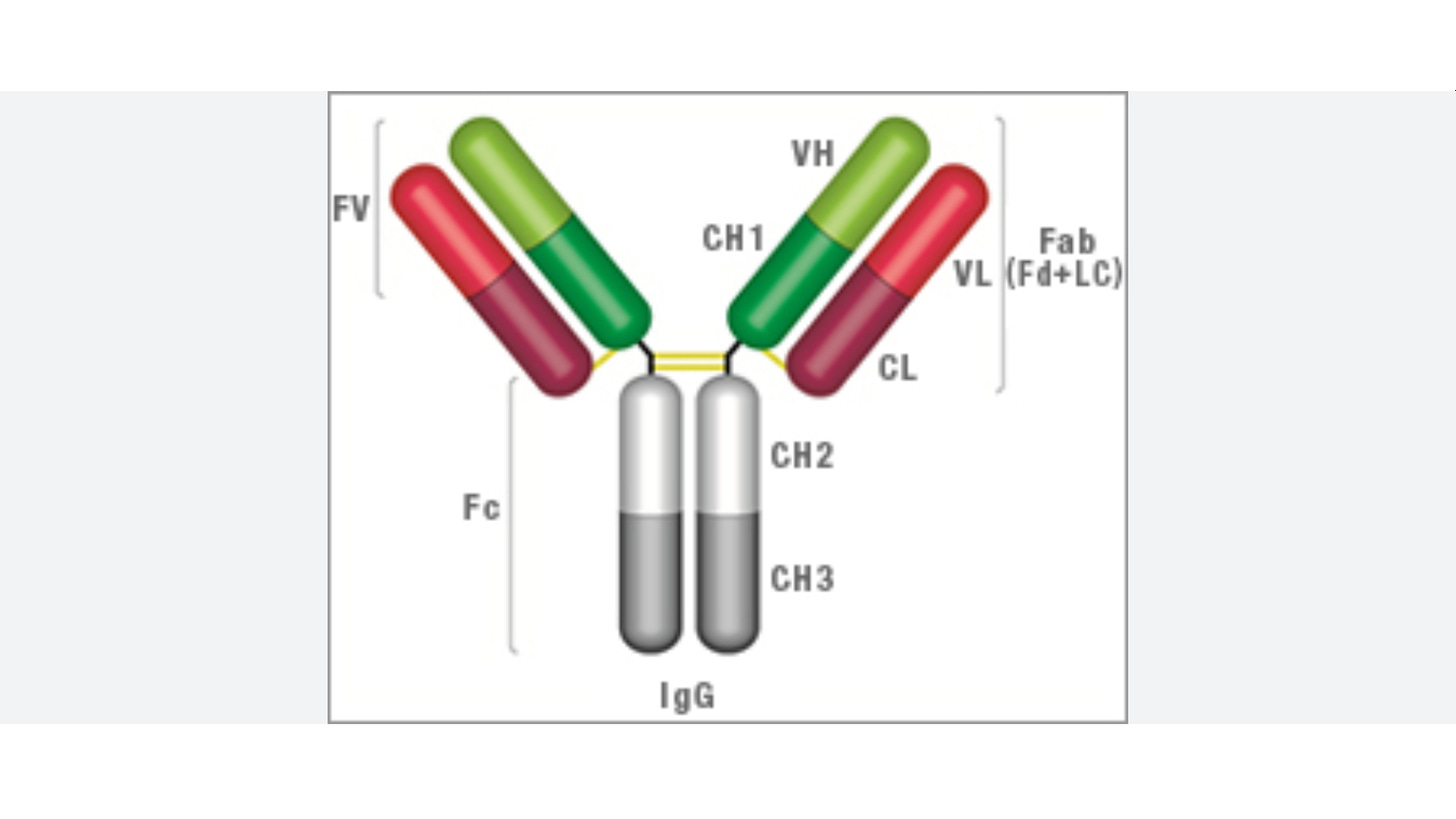
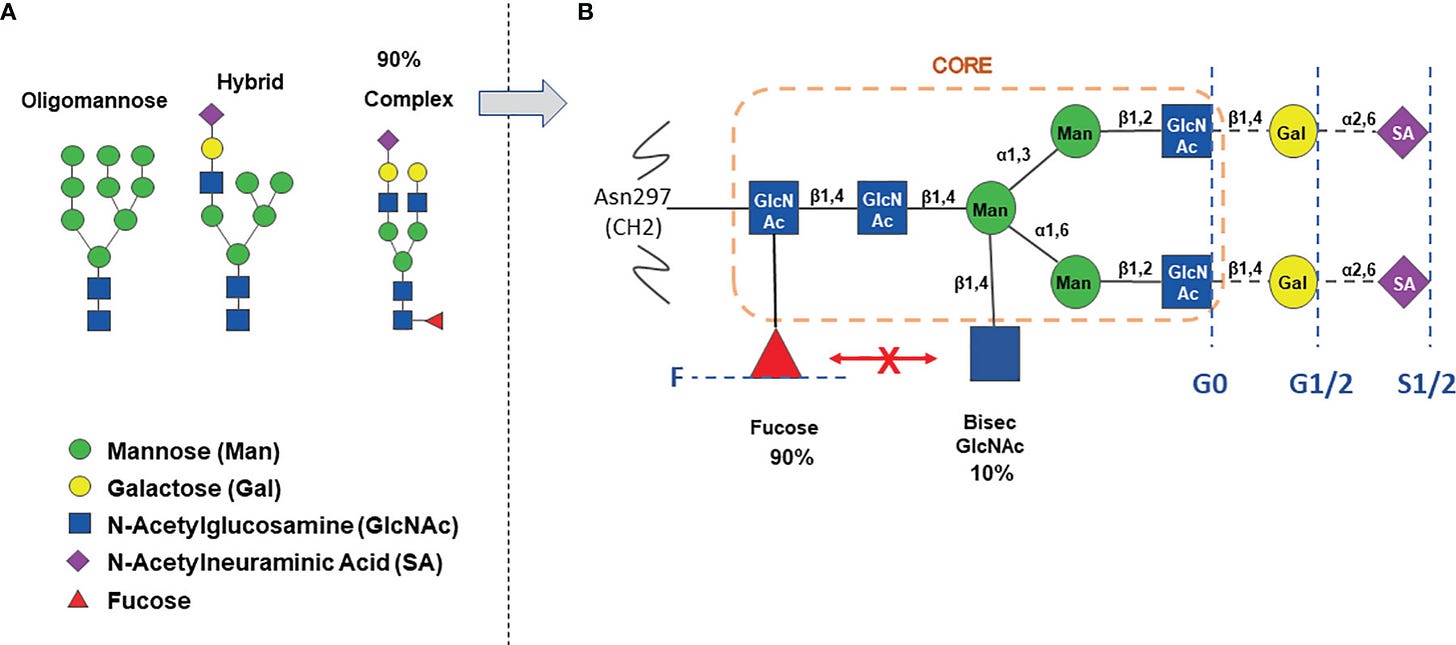
N-glycosylation site is present at conserved Asparagine 297 (Asn 297) in the CH2 domain, that interacts with FcɣRs. 20-30% of IgGs also bear N-glycans on Fab arms.
Here is a antibody showing CH2 domain
Proteins are sequences of amino acids of which Asparagine is one. I’m guessing 297 means its the 297th amino acid in the antibody which is a protein, but I dont know for sure
A glycan refers to the carbohydrate portion of a glycoconjugate, such as a glycoprotein
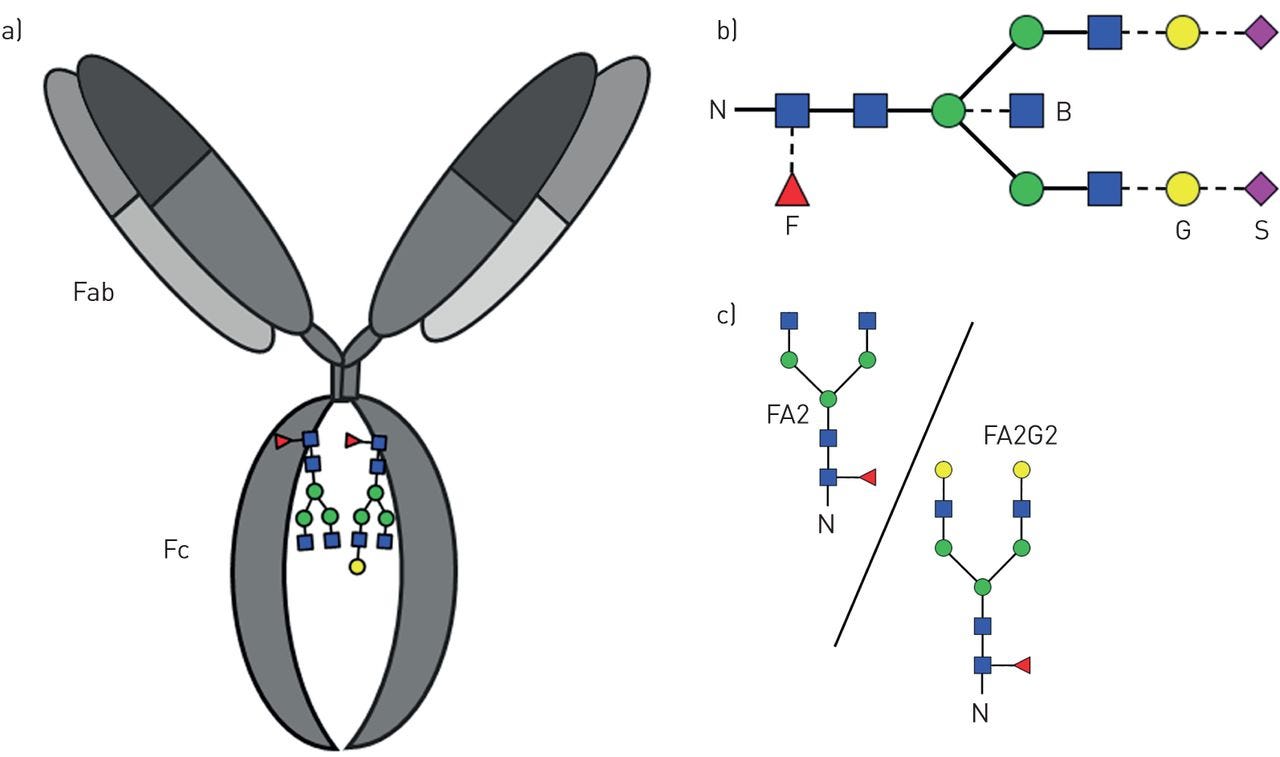
All human antibody classes are post-translationally modified by carbohydrates. The resulting glycans take on many divergent structures and can be attached in an N-linked or O-linked manner, and are distinct by antibody class, and by position on each antibody. Many of these glycan structures on antibodies are capped by sialic acid. It is well established that the composition of the N-linked glycans on IgG exert a profound influence on its effector functions.
Here is a another picture of an antibody showing Fc location of glycans such Galactose (G) , Sialic acid (S) and Fucose (F)
The Red triangle is Fucose
The presence of fucose on IgG1 Asn-297 N-linked glycan is the modification of the human IgG1 Fc structure with the most significant impact on FcɣRIII (also known as CD 16) affinity. It also significantly enhances the efficacy of antibody dependent cellular cytotoxicity (ADCC) by natural killer (NK) cells
Ok, lets talk about how antibodies interact with our cells
A picture worth a thousand words. Its that simple. Cells which interact with the Fc of the antibody are called Effector Cells. They have Fc Receptors. Not all Receptors bind with the Fc of all IgG. It depends on the IgG class and type and glycans
All of the Fcγ receptors (FcγR) belong to the immunoglobulin superfamily and are the most important Fc receptors for inducing phagocytosis of opsonized (marked) microbes. This family includes several members, FcγRI (CD64), FcγRIIA (CD32), FcγRIIB (CD32), FcγRIIIA (CD16a), FcγRIIIB (CD16b), which differ in their antibody affinitiesdue to their different molecular structure. For instance, FcγRI binds to IgG more strongly than FcγRII or FcγRIII does.
The Fc-gamma receptors differ in their affinity for IgG and likewise the different IgG subclasses have unique affinities for each of the Fc gamma receptors.
These interactions are further tuned by the glycan (oligosaccharide) at position CH2-84.4 of IgG. For example, by creating steric hindrance, fucose containing CH2-84.4 glycans reduce IgG affinity for FcγRIIIA. In contrast, G0 glycans, which lack galactose and terminate instead with GlcNAc moieties, have increased affinity for FcγRIIIA.
https://en.m.wikipedia.org/wiki/Fc_receptor
So anyways, its complicated but you get the general idea, not all IgG does the same stuff.
Back to fucose. An α-1,6 fucose residue (core fucose) is present in 90% of complex type IgG N-glycans .
In the last 10-15 years, removal of IgGs Fc core fucose has been shown to be an important method to enhance ADCC by therapeutic IgG1 mAbs. This can also happen naturally during viral infections, especially with enveloped viruses like HIV, Dengue and Sars-Cov-2
Antibodies without Fc core fucose are called afucosylated
https://www.frontiersin.org/articles/10.3389/fimmu.2022.929895/full
.
Afucosylated IgG responses were often accompanied by increased galactosylation
The level of afucosylation is not predetermined by general host factors such as genetics but is rather stochastic or multifactorial, with the specific triggers remaining obscure.
Hepatitis B Ag-specific antibodies in individuals who cleared a natural infection show lowered Fc fucosylation compared with that in individuals who received protein subunit vaccination. This strongly suggests that a specific context for the antigenic stimulus is required for afucosylated IgG responses.
Total IgG1-Fc galactosylation and sialylation levels were significantly lowered in the ARDS patients, which was perhaps a reflection of a slight age difference between these two groups [non-ARDS donors median age (IQR) 49 (40 to 55) years versus ARDS patients 60 (55–63) years
Both Fc galactosylation and sialylation decrease with age. Increased galactosylation and sialylation of antigen-specific IgG1-Fc increases complement activity by approximately three- to fourfold.
Fc galactosylation further enhances affinity of afucosylated IgG to FcγRIII by approximately twofold .
Further, accumulating evidence strongly suggests that the primary and major biologically relevant change in IgG-Fc glycosylation is the lack of core fucose.
Afucosylated IgG have a 20- to 40-fold increase in affinity to FcγRIIIa, which is often accompanied by an absolute change from no cellular response to strong phagocytic and ADCC responses upon afucosylation
The lowered Fc fucosylation in the anti-S responses of the ARDS patients suggests a pathological role through FcγRIIIa, similar to what has previously been proposed for dengue .
[They are describing Antibody Dependent Enhancement which has also been observed with Dengue Vaccines]
In dengue, non-neutralizing antibodies that were formed to previous infections of other dengue serotypes also tend to have low amounts of core-fucosylated IgG. Because they are incapable of preventing infection, they lead to aggravated dengue hemorrhagic fever because of FcγRIIIa-mediated overreactions by immune cells
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7919849/
Since effector function(s), including antibody-dependent cell-mediated cytotoxicity (ADCC), require the Fc portion to be glycosylated, reduced ADCC activity antibodies can be obtained through aglycosylation of the human IgG1 isotype.
An alternative is to switch to an IgG4 isotype in which the glycosylated antibody is known to have reduced effector function relative to glycosylated IgG1 antibody. ADCC activity of glycosylated IgG1 antibodies is sensitive to the fucosylation status of the Fc glycan, with both in vitro and in vivo ADCC activity increased upon fucose removal ("afucosylation").
The effect of afucosylation on activity of IgG4 antibodies is less well characterized, but it has been shown to increase the in vitro ADCC activity of an anti-CD20 antibody. Here, we show that both in vitro and in vivo activity of anti-CD20 IgG4 isotype antibodies is increased via afucosylation.
Binding of antibodies to FcγRIIIa on natural killer (NK) cells enables the immune system to target designated cells for destruction. ADCC can be employed in therapeutic agents to remove pathologic cells including malignant tumor cells. For example, non-Hodgkin's lymphoma (NHL) is effectively treated with a combination of a chemotherapy regimen and administration of the anti-CD20 antibody rituximab.
Although in general IgG4 isotype antibodies have reduced capacity to support in vitro ADCC, in vivo target cell depletion activity in humans has been noted with an IgG4 version of an anti-CD52 antibody.13 As with IgG1 isotype antibodies produced in CHO cells,there could be variability in the fucose levels on IgG4 antibodies.
Afucosylation has been demonstrated previously to increase the in vitro ADCC activity of an anti-CD20, IgG4 isotype antibody. Here, we show that afucosylation does increase the in vivo B cell depletion activity of an IgG4 isotype anti-CD20 antibody, suggesting that, for some IgG4 antibodies produced in CHO, it may be necessary to monitor fucosylation status in order to limit cell killing activity
So lets get on with the paper
https://www.frontiersin.org/articles/10.3389/fimmu.2022.929895/full
So with all the talk about IgG and fucosylation, perhaps the most important part of the paper is related to IgA
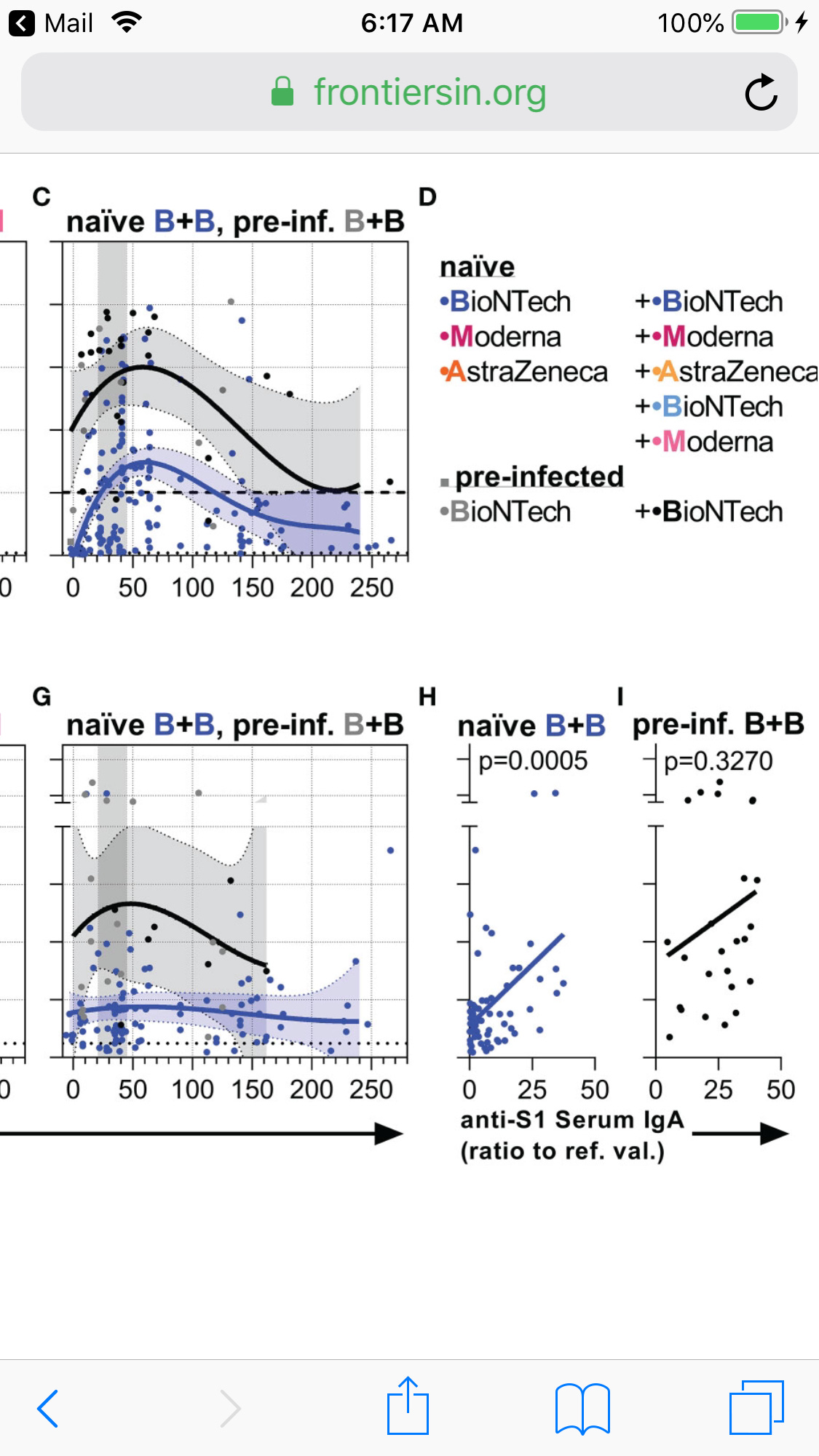
Pre-infected individuals vaccinated with BNT162b2 reached and maintained higher anti-S1 IgA levels both in serum and saliva over time compared to naïve individuals vaccinated with BNT162b2
Anti-S1 saliva IgA levels correlated in pre-infected as well as naïve vaccinees with anti-S1 saliva J-chain levels (Figure S4) suggesting that the anti-S1 salivary IgA is mostly dimeric J-chain-coupled secretory (s)IgA in all groups.
While anti-S1 salivary IgA levels correlated with anti-S1 serum IgA levels in naïve vaccinees, they did not display such a significant correlation in pre-infected ones . The findings suggest a proper, but more decoupled re-activation of local respiratory and systemic S1-reactive IgA+ B cells in pre-infected vaccinees.
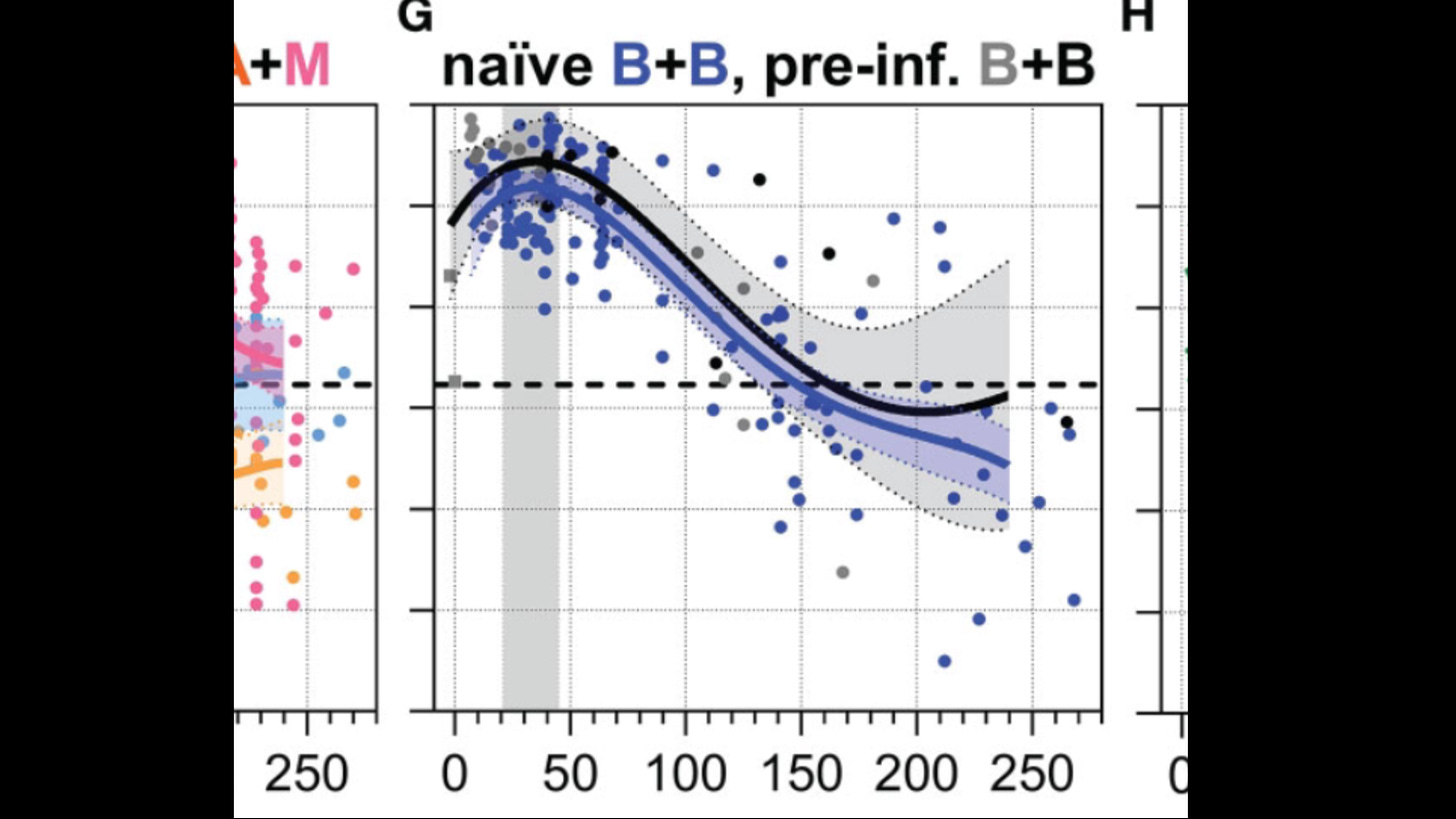
Serum IgA top, Serum IgA bottom vs days after second shot
Pre-Inf Black, naive Blue
Naïve and pre-infected vaccinees that received two BNT162b2 immunizations were separated between day 100 and 170 as follows: pre-infected vaccines showed higher anti-S1 serum and saliva IgA as well as anti-S IgG1 bisection and galactosylation levels, whereas naïve vaccines showed higher anti-S1 IgG4 and anti-S IgG1 fucosylation levels
IgA is more important to stopping respiratory infections early and naturally infected have more of it than naive Vaccinated. Too bad they didn’t include any unvaccinated in the study.
BNT162b2-vaccinated, pre-infected individuals showed a comparable anti-S IgG1 sialylation and a slightly higher anti-S IgG1 galactosylation course to naïve individuals vaccinated with BNT162b2
So back to the glycan discussion. Naive vaccinated showed higher levels of fucosylation and galactosylation anti-S IgG1 and higher anti-S1 IgG4
We assume higher anti-S1 IgG4 is bad, but what about higher fucosylation and galactosylation anti-S IgG1. Does that reduce IgG1 effector function?
Remember, Both Fc galactosylation and sialylation decrease with age, as does your overall immune system. Increased galactosylation and sialylation of antigen-specific IgG1-Fc increases complement activity by approximately three- to fourfold, which is a good thing
Note at the end the pre-infected is trending up on galactosylation while naive is trending down
So I don’t know if that is important. The authors didn’t make much of it , but we know that doesn’t mean much
Back to IgG4. I found this in another paper
Compared to other IgG isotypes, IgG4 is only weakly interacting with Fcγ receptors and linked to anti-inflammatory processes via Fab-arm exchange and clearance It is likely that the decrease in galactosylated Fc glycans of IgG4 in sarcoidosis and SA is linked or a response to lung remodelling. However, if the reduction in IgG4 galactosylated Fc glycans result in an active pro-inflammatory effect similar to what would be expected from IgG1 needs to be explored further.
https://openres.ersjournals.com/content/4/3/00033-2018
The author is wondering if reduction in IgG4 galactosylated Fc glycans can result in an active pro-inflammatory effect similar to what would be expected from IgG1
What if the lower galactosylation of the naive vaccinated who have the most IgG4 is compensating for the anti inflammatory effect of IgG4
Food for thought.
Now for more Afucosylated findings
The findings reveal that the level of afucosylated anti-spike antibodies increases transiently in infection-naïve individuals after the first vaccine dose.
In infection-naïve individuals, about 25% of anti-spike IgG1 Fc were found to be afucosylated initially after administration of the first vaccine dose. However, the level decreased gradually with time.
In individuals with previous infection, about 2 – 10% of anti-spike IgG1 Fc were found to be afucosylated before vaccination. The level increased slightly after vaccination. The level of afucosylated anti-spike antibodies remained significantly lower in previously infected individuals after vaccination compared to that in naïve individuals
IgG lacking core fucosylation at this position initiates enhanced antibody-dependent cellular cytotoxicity by increased affinity to the Fc receptor FcRIIIa.
Note: below pic not from the paper
Larsen et al. report that COVID-19 patients with severe symptoms have increased levels of anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG afucosylation compared with patients with mild disease.
Here, we report that afucosylated IgG (approximately 6% of total IgG in humans)are specifically formed against enveloped viruses but generally not against other antigens. This mediates stronger FcγRIIIa responses but also amplifies brewing cytokine storms and immune-mediated pathologies
The afucosylation of anti-S IgG may contribute to the exacerbation of COVID-19 in a subset of patients, resulting in ARDS. Thus, although they can be protective, antibodies potentially behave as double-edged swords and may contribute to the observed cytokine storm
Critically ill COVID-19 patients, but not those with mild symptoms, had high concentrations of afucosylated IgG antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), amplifying proinflammatory cytokine release and acute phase responses
Fortunately, the concern with afucosylated antibodies and vaccination seems limited to the COVID naive after the first dose. If infected shortly after vaccinating one could experiences serious disease due to afucosylated antibodies produced after vaccination. And we know that after the first and 2nd doses the risk of getting infected increased due to transient immune suppression. Of course, such cases go down as unvaxxed if infected in the first 14 days after the vax.
At this point in the Pandemic, is anyone unvaxxed and COVID naive? Seems unlikely, so this was perhaps a problem early on but unlikely to be an issue now
II- Cellular Immunity (T-Cells)
Before I get into the paper Alex Berenson posted on, it might be helpful to review basic immunology. If you want to skip this and scroll down, then do so.
Immunology 101
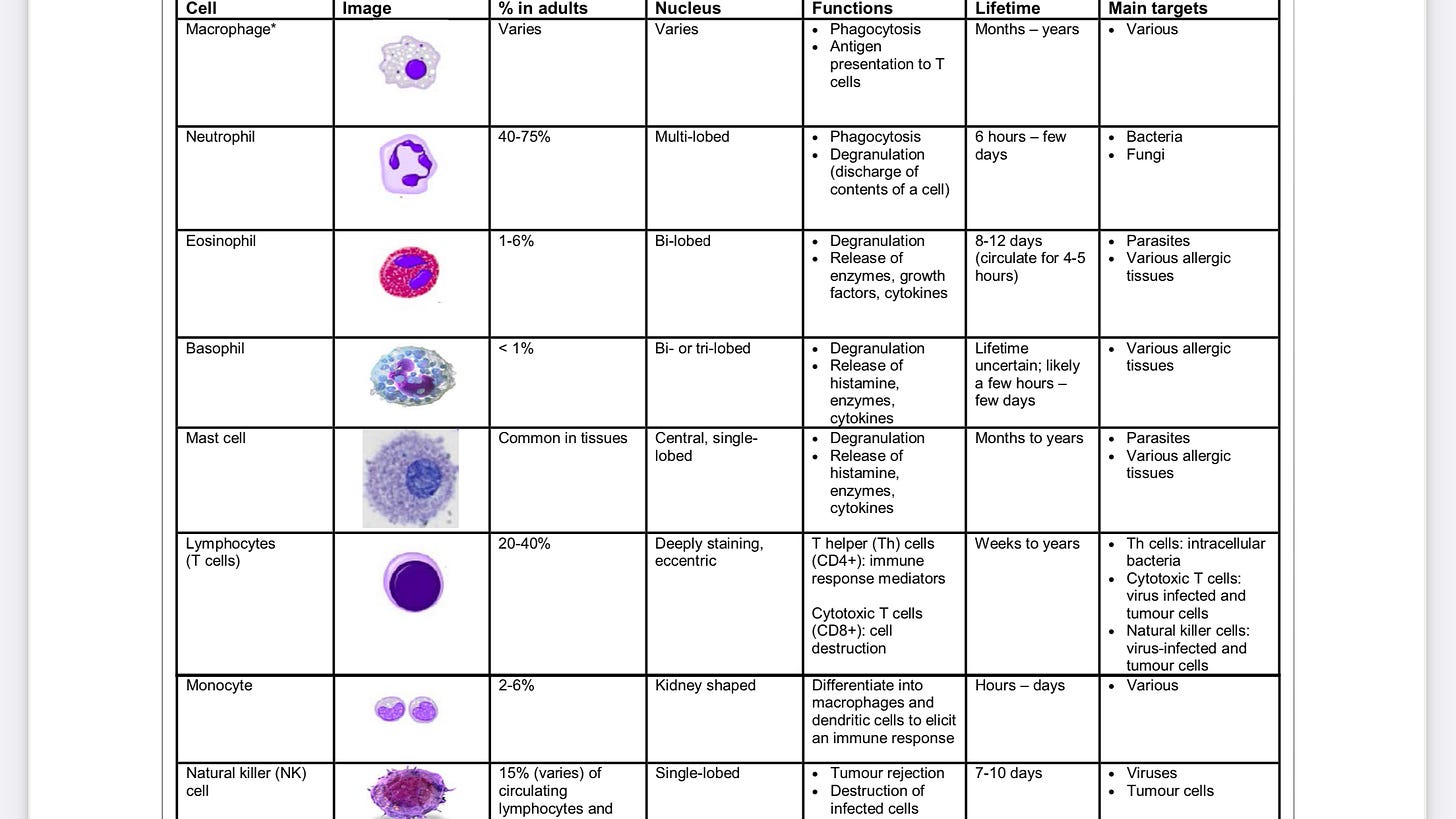
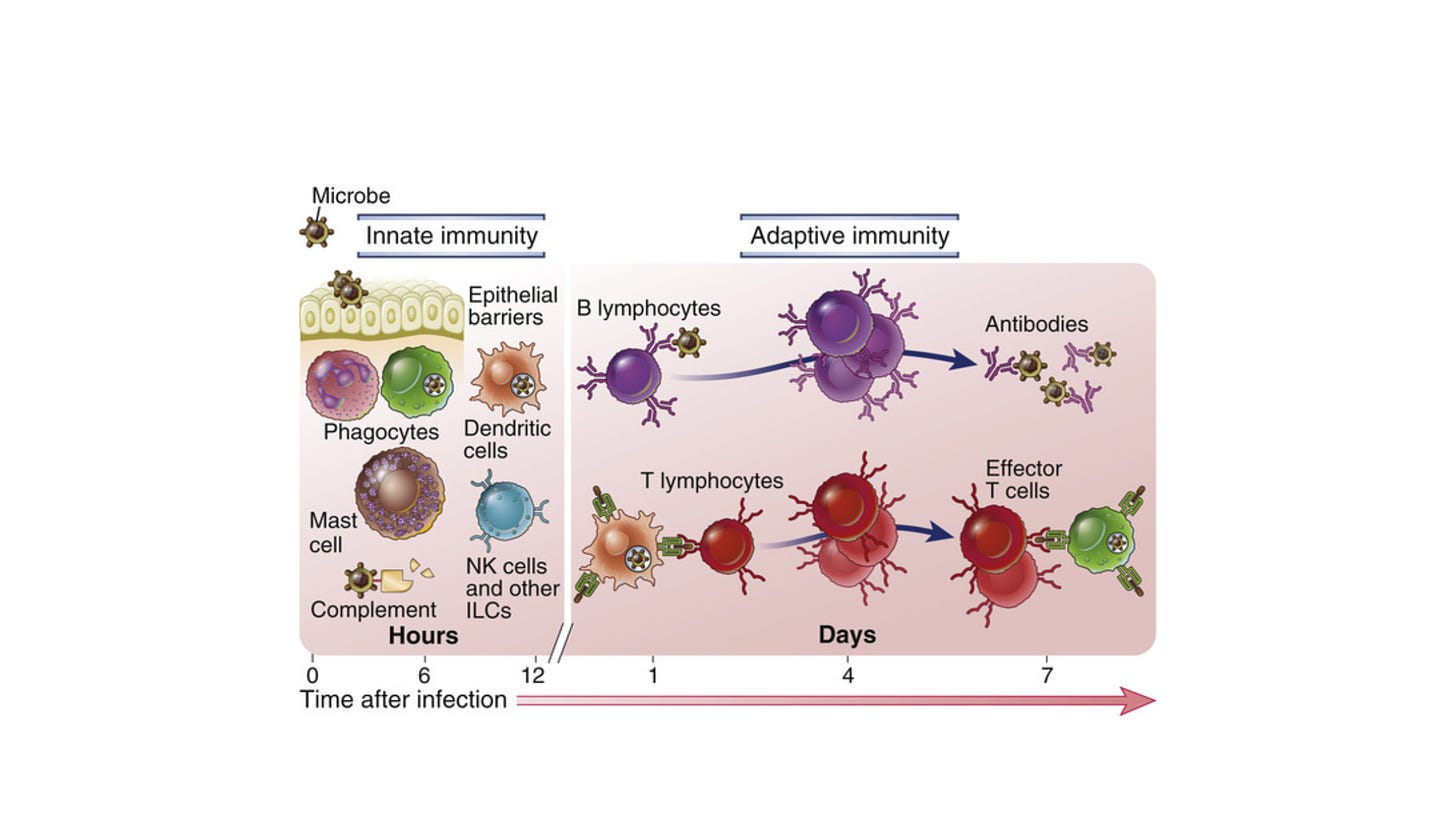
When the body detects a novel invading virus, the first line of immunological defense is the innate immune system. This arm is initiated immediately and includes neutrophils, macrophages, dendritic cells, and natural killer cells. Innate immunity activates inflammation, recruits immune cells to the infection site, activates complement, phagocytoses foreign bodies, and activates the adaptive immune response.
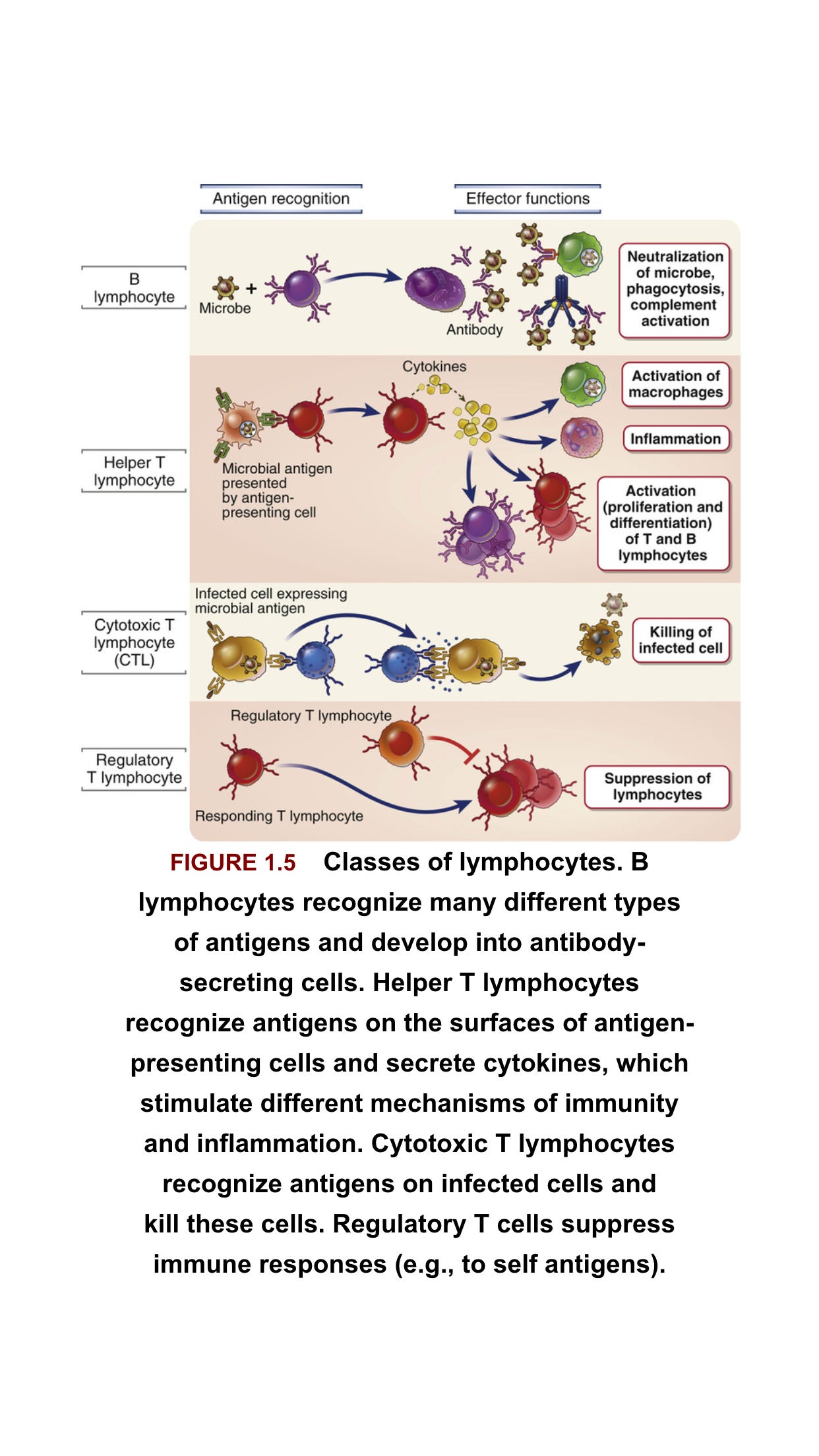
The adaptive immune response is antigen specific and takes longer to develop. It consists of humoral immunity (antibodies) and cell-mediated immunity (T cells). In the humoral response, B cells are activated to secrete antibodies, which can bind to extracellular virus and neutralize it, that is, prevent it from infecting target cells. There is also a non-neutralizing antibody response, where binding of antibody to antigen can result in enhanced phagocytosis or activation of the complement cascade.
T cells respond to intracellular virus and largely consist of CD4+ T cells and CD8+ T cells. Viral protein that is produced in the cytosol, or that is phagocytosed into lysosomes, is digested by the proteasome, and short peptides are translocated to the endoplasmic reticulum where they bind to major histocompatibility complex (MHC) molecules. Cytosolic-derived peptides bind to MHC Class I and are presented on the cell surface to CD8+ T cells.
Virus phagocytosed by professional antigen-presenting cells (APCs) produces peptides that bind to MHC Class II, which are presented on the cell surface to CD4+ T cells.
CD8+ T cells, also known as cytotoxic T lymphocytes (CTLs), recognize virus-infected cells presenting the same peptide-MHCs that activated them in the first place and destroy the cell via granzyme B or perforin, limiting viral spread.
CD4+ T cells, which are activated by professional APCs, include several specific groups with different functions. Broadly speaking, T helper 1 (Th1) cells enhance the CTL response; Th2 cells enhance the antibody response and anti-parasitic immunity; T follicular cells (Tfh) enhance the antibody response; Th17 cells promote inflammation but can also cause autoimmunity; and regulatory T cells (Tregs) act to maintain homeostasis (a balance between immunogenicity and immune tolerance) and inhibit autoimmunity .
[Th2 skewed immune response associated with ADE]
If not more important, in controlling infection. Both CD8+ and CD4+ T cells are elicited earlier and are associated with milder disease, than antibodies, and T-cell activation appears to be necessary for control of infection.
Variants of concern (VOCs) such as Omicron have escaped the neutralizing antibody responses after two mRNA vaccine doses, but T-cell immunity is largely intact. The breadth and patient-specific nature of the latter offers a formidable line of defense that can limit the severity of illness, and are likely to be responsible for most of the protection from natural infection or vaccination against VOCs which have evaded the antibody response.
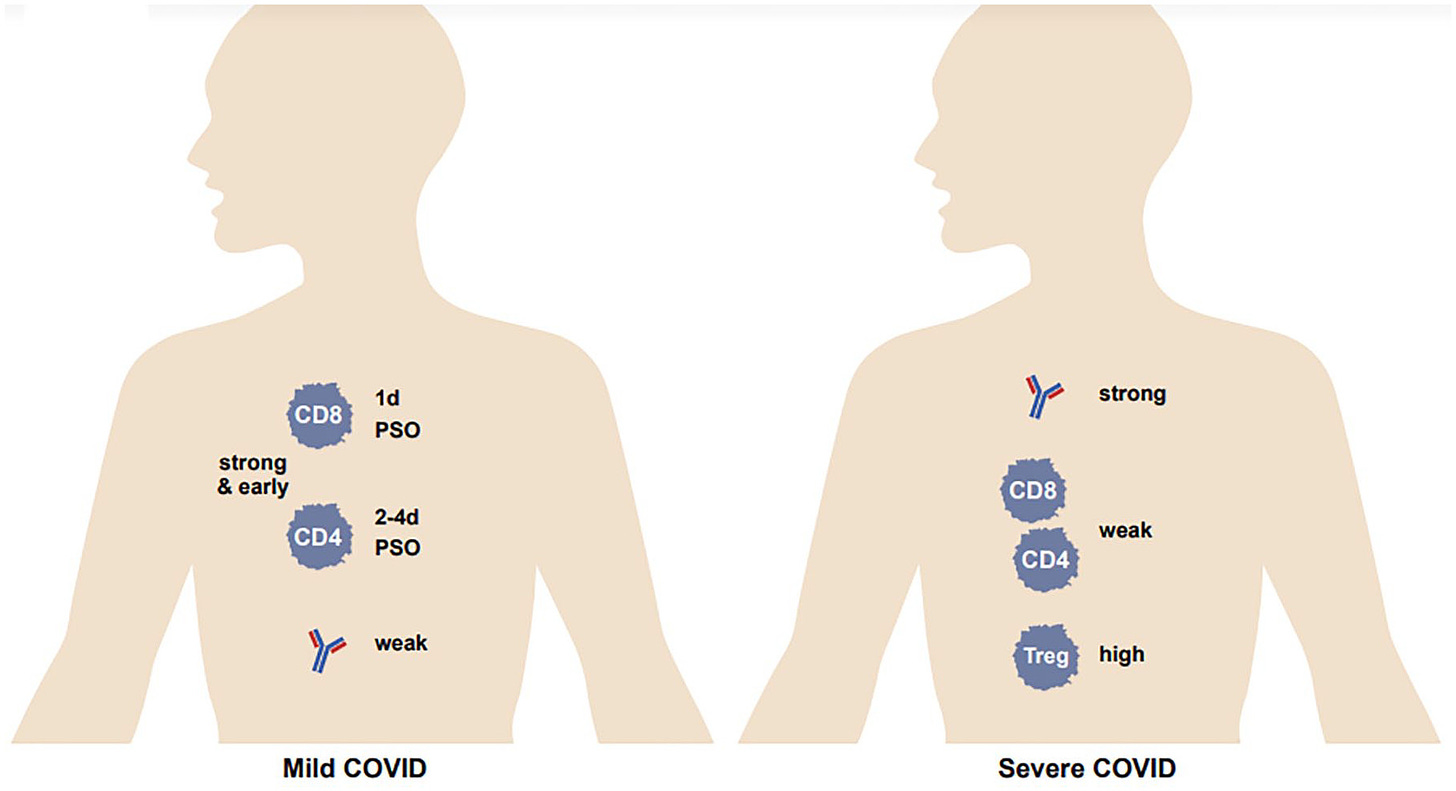
It has been widely recognized that a strong antibody response is associated with severe COVID-19, whereas for T cells it is the opposite – a strong and early response is associated with mild disease. On the other hand, there is minimal induction of T cells in moderate to severe disease,and an impairment in the T-cell response in death. (However, some reports have observed opposite results....) A high level of Tregs (suppressive T-cell response) was associated with severe COVID
Sars-Cov-2
T cells are induced in natural SARS-CoV-2 infection extremely rapidly: CD4+ T cells can be detected at 2–4 days post-symptom onset (PSO), and CD8+ T cells as early as 1 day PSO, whereas seroconversion (generation of antibodies) occurs between 5 and 15 days PSO. Many studies have examined the duration of T-cell immunity after natural infection, as well as that of antibodies and memory B cells. CD4+ and CD8+ T cells have a half-life of 200 days. Another study observed a decline in CD4+ and CD8+ T cells after natural infection, with a half-life of 3–5 months. Chen et al. report that the level of spike-specific CD4+ T cells does not change by 7 months after infection. A detectable T-cell response to SARS-CoV-2 antigens was present in 93% and 92% of patients at 6 months and 12 months after disease onset, respectively.
About 95% of COVID-19 convalescents had detectable NAbs from 6 months to 12 months after the onset of disease
Virus-specific CD4+ T cells dominate the T-cell response, with a smaller contribution from virus-specific CD8+ T cells. In 100% and 70% of convalescent patients, SARS-CoV-2-specific CD4+ T cells and CD8+ T cells could be detected, respectively.
Within the CD4+ T-cell population, Th1 and Tfh subpopulations predominate. Th1 cells produce interferon-γ (IFN-γ) and other cytokines which help control viral infection, as well as help CD8+ T cells. Tfh cells help elicit virus-specific NAbs. There is evidence that skewing toward a Th2-dominant response is associated with poor viral control
In persistent viral infection, T cells can undergo a state of exhaustion. CD8+ T-cell exhaustion involves the loss of interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α), and IFN-γ production and a gradual loss of proliferative capacity. CD4+ T cells can also undergo exhaustion, exemplified by a loss in effector function. A high level of inhibitory receptors are expressed, including lymphocyte-activation protein 3 (LAG3), cytotoxic T lymphocyte-activation antigen 4 (CTLA-4), and T-cell immunoglobulin and mucin-domain containing protein 3 (TIM-3)
Nonetheless, it should be pointed out that most studies on T-cell exhaustion have been with chronic viruses like HIV; the vast majority of COVID-19 patients clear their infection, so T-cell exhaustion must not usually be due to persistent virus, except for those who are immunocompromised in whom virus can exist for a long duration.
Surprisingly, although SARS-CoV-2 is a relatively new human coronavirus, many studies have found preexisting T cells to the endemic cold coronaviruses which also cross-reacted to SARS-CoV-2 in a subset of individuals. Fifty-two COVID-19 household contacts were sampled at the earliest timepoint after exposure, and those who remained polymerase chain reaction (PCR)-negative for virus had statistically significantly higher T cells cross-reactive to both human endemic coronavirus and SARS-CoV-2 nucleocapsid (but not to spike) than those who became PCR-positive.
Sagar et al. found that individuals who had a recent history of human coronavirus infection had lower rates of intensive care unit admissions and better survival after acquiring SARS-CoV-2 infection. In fact, SARS-CoV-2 cross-reactive CD4+ T cells were found in as many as 66% of healthy, unexposed individuals
[In China and much of Asia exposure to common coronavirus was high due to high population density, so they no doubt had higher rates of natural immunity]
Fine-needle aspiration of draining lymph nodes in patients who had received the BNT162b2 vaccine detected spike-specific T cells being maintained at constant frequencies out to 6 months post-vaccination. Interestingly, in neuro-COVID long-haulers, that is, patients with post-acute sequelae of SARS-CoV-2 infection (PASC), there were decreased spike-specific but increased nucleocapsid- and membrane-specific T cells compared with healthy convalescents.
Numerous studies reported that convalescents who received their first mRNA vaccine dose had similar levels of T cells induced as naïve patients who received their second dose, and that the second dose does not further induce T cells in convalescent patients
While the VOCs have significant reductions in susceptibility to the vaccine-induced humoral response, it appears that the cellular response is less affected. A study on B.1.1.7 (Alpha) and B.1.351 (Beta) variants found no observable differences in CD4+ T-cell activation in comparison with the vaccine strain. Another study observed that the neutralizing antibody response against B.1.351 was more reduced than that against B.1.1.7, but that the T-cell response was directed against epitopes that were conserved between the vaccine strain and these two VOCs. Neidleman et al. reported that the overall T-cell response to the ancestral (vaccine) strain, B.1.1.7, and B.1.351 were similar; but spike-specific T cells from convalescent vaccinees had more long-term persistence and better homing to the respiratory tract, including the nasopharynx, than naïve vaccinees. Later studies incorporated the Delta (B.1.617.2) variant. Both Woldemeskal et al. and Jordan et al. found that the overall T-cell response and the CD4+ and CD8+ T-cell response to BNT162b2, respectively, were virtually the same against Delta as the ancestral strain.
Data on Omicron are more sparse, since this VOC is newer, but preliminary observations are that most T-cell epitopes against the ancestral strain are conserved in Omicron, suggesting that even this variant might not have escaped the T-cell arm of the immune response to the vaccine and to prior infection. Still, a recent report found that 20% of individuals examined had a T-cell activity against Omicron that was reduced by more than 50%.
T-cell response to SARS-CoV-2 seems to be less affected by variants than the antibody response. First, there are more potential T-cell epitopes than antibody epitopes, because the former can sample across all viral proteins, not just external ones. Greater breadth means less chance for a variant to escape all of them – and even any of them, because mutation away from one cannot be selected for unless it can mutate away from, not necessarily all, but enough comprising the overall T-cell response.
Second, T-cell epitopes are highly diverse from individual-to-individual, because they are restricted by the MHC (HLA) haplotype. Even if, in a hypothetical scenario, a variant arises within the quasispecies (the diverse population of minor or unique variants within a single individual) to escape that patient’s T-cell immunity, it is likely to be fully sensitive to the T-cell response of the next individual, and thus would be stopped dead in its tracks.
All of these observations underscore a valuable lesson: it perhaps will prove to be highly important, especially in the long run, to design vaccines that specifically and potently target T cells. We do not suggest that antibody vaccines or antibody components to vaccines should be abandoned, but rather supplemented. There are ways to directly target T cells, such as epitope vaccines, vaccines that incorporate internal proteins, or the use of T cell-activating adjuvants. T cells often get short shrift in the vaccine world, and antibodies often not only take center stage but can even lead people to lose sight of T cells; this is likely to be a grievous mistake, but one that is certainly correctable.
Antibodies against SARS-CoV-2, as with many other respiratory viruses, are afforded an outsized emphasis, both by the lay public and by virologists, as exemplified by, for example, the near-immediate analysis of the humoral response when a new variant arises. This is not just due to the relative ease with which neutralization assays can be implemented, but also to the dogma that antibodies stand first in line to prevent infection altogether. However, as mentioned above, T cells arise much earlier after natural SARS-CoV-2 infection than do antibodies; and high T-cell activity is associated with controlled viral infection, whereas the opposite has proven to be the case for antibodies.
Indeed, that the current vaccines are seemingly unable to neutralize Omicron (the neutralizing antibody titer is down 22-fold after two doses), but can still greatly reduce severe disease, and often even symptomatic infection, is likely attributable in large part to the cellular immune response. Also of note is the few patients with agammaglobulinemia (unable to make IgG) who have been studied who fully control SARS-CoV-2 infection.
https://journals.sagepub.com/doi/full/10.1177/25151355221115011
During acute viral infection, naive CD8+ T cells that recognize antigens presented on MHC-I by their T-cell receptors (TCRs) are activated and undergo clonal expansion and differentiation into effector CD8+ T cells Effector CD8+ T cells produce cytokines, including IFN-γ and tumor necrosis factor (TNF), and directly kill target cells . In the subsequent contraction phase following antigen clearance, a small proportion of effector CD8+ T cells differentiate into memory CD8+ T cells . Memory CD8+ T cells rapidly exert effector functions upon antigen re-encounter, playing a crucial role in host protection during reinfection
On the other hand, when antigens persist in chronic viral infection or cancer, the development of memory CD8+ T cells fails, and the effector functions of CD8+ T cells become impaired . This state of CD8+ T cells is called “exhaustion.” CD8+ T-cell exhaustion was first reported in a previous study using a mouse model of chronic lymphocytic choriomeningitis virus (LCMV) infection . LCMV-specific CD8+ T cells that are continuously stimulated by antigens exhibit impaired effector functions and limited proliferation compared to conventional memory CD8+ T cells . These findings have also been observed in human patients with chronic viral infection or cancer . T-cell exhaustion is evidently the main mechanism underlying immune dysfunction during chronic viral infection and cancer and virus antigen-specific and tumor antigen-specific CD8+ T cells exhibit features of T-cell exhaustion and dysfunction . CD8+ T cell exhaustion is now considered a distinct differentiation state of CD8+ T cells, with several key features
[consider that repeated boosting and reinfections, not to mention persistence of spike protein mimics chronic infection]
https://www.nature.com/articles/s41423-021-00750-4
THE MICE STUDY (its almost over)
https://www.sciencedirect.com/science/article/pii/S2589004222017515
[Remember this was was using the China subunit RBD-Spike protein vaccine, not mRNA]
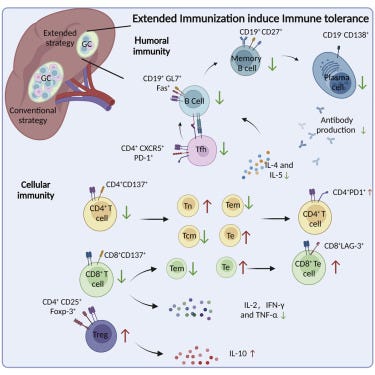
Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice
Multiple vaccine boosters after the conventional vaccination course significantly decreased RBD-specific antibody titers and serum neutralizing efficacy against the Delta and Omicron variants, and profoundly impaired CD4+ and CD8+T cell activation and increased PD-1 and LAG-3 expressions in these T cells. Mechanistically, we confirmed that extended vaccination with RBD boosters overturned the protective immune memories by promoting adaptive immune tolerance.
PD-1 is a protein cells express that cause immune cells like cytopathic CD8+ T-Cells to become lazy so PD-1 Inhibitors are a popular cancer immune therapy
Foreign antigen stimulation can induce immune tolerance, which is manifested as inability or low efficiency to produce antigen-specific antibodies and to activate effector T cells
IgG1 titer in mice immunized by multiple boosters was significantly lower than that without booster. These results suggested that the RBD vaccine could stimulate the production of RBD-specific antibodies with a dominance of the Th2-type
[this is not good news for the Chinese people, but not sure it applies to mRNA so I don’t emphasize the studies antibody results too much]
To investigate the effect of vaccine boosters on CD8+T cells, we studied the secreted levels of the effector cytokines one week after the last immunization. Serum concentrations of IL-2, IFN-γ and TNF-α were significantly increased by both immunization courses, indicating a functional activation of CD8+T cells . But the extended vaccination profoundly reduced the secretion of all three cytokines than the conventional [2 dose] immunization
It has been reported that repeated antigen stimulation induces the exhaustion of CD8+T cells; therefore, we tested whether there were any differences in exhaustion marker levels between two immunization courses. We found that the cell surface expressions of PD-1 and LAG-3 on CD8+T cells from mouse splenocytes were evidently higher in the extended vaccination group, comparing to either the conventional group or the PBS control .
These data indicated that continues administration of RBD booster vaccines could lead to reduced CD8+T cell activation with increased exhaustion. Overall, our findings evidenced the potential risk of adaptive immune tolerance from prolonged course of immunization with homologous vaccine boosters, and suggested that the applications of multiple booster vaccines with protective intent should be preceded with caution.
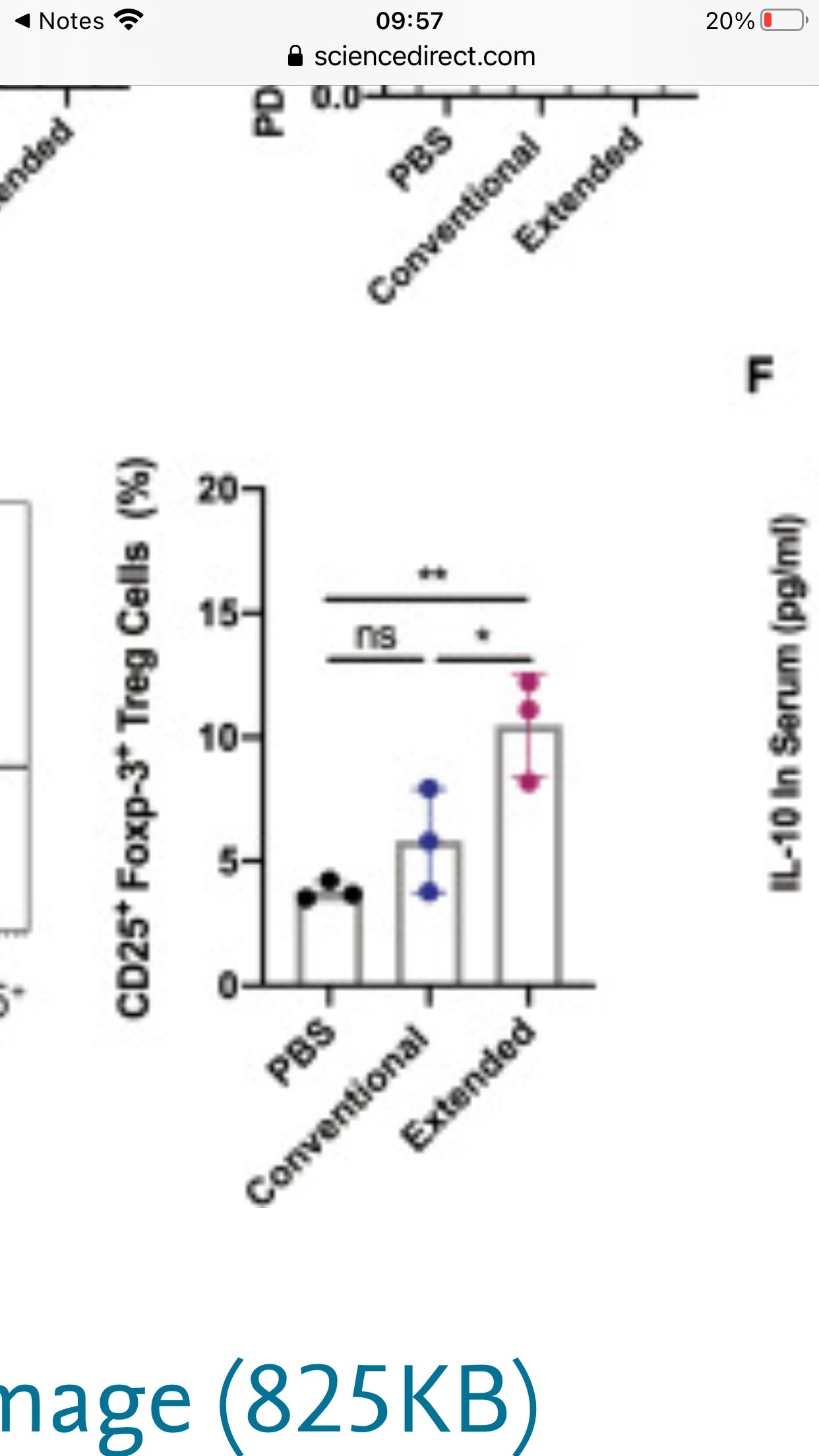
An increased percentile of Treg cells was also observed, accompanied by significant elevation of IL-10 production. Together, we provided crucial evidence that repetitive administration of RBD booster vaccines may negatively impact the immune response established by a conventional vaccination course and promote adaptive immune tolerance.
[Thats quite an increase in Tregs which act to dial down your immune response]
Although RBD subunit vaccines cannot entirely represent inactivated or mRNA vaccines, especially in antigen delivery way. A recent report in The New England Journal of Medicine demonstrated that a fourth mRNA vaccination of healthy young health care workers only shows marginal benefits (Regev-Yochay et al., 2022). Whether extended vaccination with other COVID-19 vaccines based on wild-type SARS-CoV-2 sequence will induce immune tolerance, further investigations are required.
We used a rodent animal model instead of primates in this study. Although the actual kinetics of immune reactivity between mice and humans is not fully understood, the Balb/c mice model has been shown to share profound similarities with humans in response to SARS-CoV-2 infections (Halfmann et al., 2022). Thus, the observed adaptive immune tolerance associated with extended booster vaccination might present important reference value, particular for the recipients of homologous vaccines.
Funny how a similar study has not been done or reported on by Pfizer or Moderna. Come on guys, whats a little mouse cost, $ 60 bucks? You could inject thousands of these little guys over and over and not have to skimp on lunch to stay profitable. Probably they did, but they won’t show anyone, even FDA. This way they can avoid the nasty stuff being obtained via FOIA
Conclusion-the immune system is remarkably complex and durable and even immunologists don’t fully understand it. I don’t think increased antigen specific IgG4 and Tregs are a great sign, but the immune system has many moving parts and especially with those who have been previously infected or otherwise in good health , I don’t think you have much to worry about, especially if you abstain from more injections and keep some IVM handy for when you come down with symptoms.
End -